
Exploring the antifungal potential of Allium sativum and Ocimum gratissimum against post-harvest fungal pathogens in mango fruits
Vol 4, Issue 2, 2023
VIEWS - 3297 (Abstract)
Download PDF
Abstract
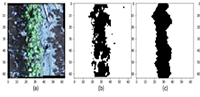
Post-harvest spoilage of fruits and vegetables caused by fungal pathogens is a serious challenge to fruit production in many parts of the world. The study was conducted to evaluate the sensitivity of fungal pathogens associated with post-harvest rot of mango fruits to crude extracts from two edible plants, Allium sativum and Ocimum gratissimum, in the study area. Five different fungal isolates were isolated from diseased mango fruits collected from fruit stores in the study area and identified as Aspergillus spp. (M1), Rhizopus spp. (M2), Fusarium spp. (M3), Penicillium spp. (M4), Fusarium spp. (M5), Penicillium spp. (M6), Aspergillus spp. (M7), and Colletotrichum spp. (M8) using radial growth rate and morphological features of the mycelia. A constant concentration of each of the crude extracts was applied to the growth media containing the growing cultures of the fungal isolates. The radial extension of the colonies for each isolate was measured along pre-marked perpendicular axes on the base of the petri dish after 24 h, and this continued for 10–14 days. It was observed that Rhizopus spp., Fusarium spp., Penicillium spp., and Colletotrichum spp. had the least growth rate when treated with the extracts.
Keywords
References
- Food and Agriculture Organization of the United Nations. Crops and livestock products. Available online: http://www.fao.org/faostat/en/#data/QCL (accessed on 30 September 2023).
- Yadav D, Singh SP. Mango: History origin and distribution. Journal of Pharmacognosy and Phytochemistry 2017; 6(6): 1257–1262.
- Kim H, Castellon-Chicas MJ, Arbizu S, et al. Mango (Mangifera indica L.) polyphenols: Anti-inflammatory intestinal microbial health benefits, and associated mechanisms of actions. Molecules 2021; 26(9): 2732. doi: 10.3390/molecules26092732
- Gold-Smith F, Fernandez A, Bishop K. Mangiferin and cancer: Mechanisms of action. Nutrients 2016; 8(7): 396. doi: 10.3390/nu8070396
- Yahia EHM. Postharvest Handling of Mango: Technical Report. Agricultural Technology Utilization and Transfer/RONCO; 1999.
- Yahaya SM, Mardiyya AY. Review of post-harvest losses of fruits and vegetables. Biomedical Journal of Scientific & Technical Research 2019; 13(4): 10192–10200. doi: 10.26717/BJSTR.2019.13.002448
- Abdulkhair WM, Alghuthaymi MA. Plant pathogens. In: Rigobelo EC (editor). Plant Growth. IntechOpen; 2016. pp. 49–59. doi: 10.5772/65325
- Ladaniya M. Citrus Fruit: Biology, Technology and Evaluation, 1st ed. Academic Press; 2010. 886p.
- Fatima N, Batool H, Sultana V, et al. Prevalence of post-harvest rot of vegetables and fruits in Karachi, Pakistan. Pakistan Journal of Botany 2009; 41(6): 3185–3190.
- Mailafia S, Olabode HO, Osanupin R. Isolation and identification of fungi associated with spoilt fruits vended in Gwagwalada market, Abuja, Nigeria. Veterinary World 2017; 10(4): 393–397. doi: 10.14202/vetworld.2017.393-397
- Karunanayake LC, Sinniah GD, Adikaram NK, Abayasekara CL. Alternatives to synthetic fungicides in controlling postharvest anthracnose and stem-end rot in mango. In: Proceedings of the III International Symposium on Postharvest Pathology: Using Science to Increase Food Availability; 7–11 June 2015; Bari, Italy. pp. 453–460. doi: 10.17660/ActaHortic.2016.1144.67
- Prusky D, Shalom Y, Kobiler I, et al. The level of quiescent infection of Alternaria alternata in mango fruits at harvest determines the postharvest treatment applied for the control of rots during storage. Postharvest Biology and Technology 2002; 25(3): 339–347. doi: 10.1016/S0925-5214(01)00169-7
- Pujari KH, Joshi MS, Shedge MS. Management of postharvest fruit rot of mango caused by Colletotrichum gloeosporiodes. In: Golding JB, Heyes JA, Toivonen PMA (editors). XXIX International Horticultural Congress on Horticulture: Sustaining Lives, Livelihoods and Landscapes (IHC2014), Proceedings of International Symposia on Postharvest Knowledge for the Future and Consumer and Sensory Driven Improvements to Fruits and Nuts; 17–22 August 2014; Brisbane, Australia. International Society for Horticultural Science; 2016. pp. 215–218.
- Goudarzi A, Samavi S, Amiri Mazraie M, Majidi Z. Fungal pathogens associated with pre‐ and post-harvest fruit rots of mango in southern Iran. Journal of Phytopathology 2021; 169(9): 545–555. doi: 10.1111/jph.13027
- Chukunda FA, Baraka RE, Azubuike P. Post-harvest diseases of mango (Mangifera indica L.) fruits in port Harcourt, Nigeria. Nigerian Journal of Mycology 2020; 12(2): 162–173.
- Sharma B, Singh BN, Dwivedi P, Rajawat MV. Interference of climate change on plant-microbe interaction: Present and future prospects. Frontiers in Agronomy 2022; 3. doi: 10.3389/fagro.2021.725804
- Chen J, Shen Y, Chen C, Wan C. Inhibition of key citrus postharvest fungal strains by plant extracts in vitro and in vivo: A review. Plants 2019; 8(2): 26. doi: 10.3390/plants8020026
- Oyetayo VO, Ogundare AO. Antifungal property of selected Nigerian medicinal plants. In: Razzaghi-Abyaneh M, Rai M (editors). Antifungal Metabolites from Plants. Springer; 2013. pp. 59–77. doi: 10.1007/978-3-642-38076-1_3
- Kahramanoğlu İ, Nisar MF, Chen C, et al. Light: An alternative method for physical control of postharvest rotting caused by fungi of citrus fruit. Journal of Food Quality 2020; 2020: 8821346. doi: 10.1155/2020/8821346
- Ugbogu OC, Emmanuel O, Agi GO, et al. A review on the traditional uses, phytochemistry, and pharmacological activities of clove basil (Ocimum gratissimum L.). Heliyon 2021; 7(11): e08404. doi: 10.1016/j.heliyon.2021.e08404
- Borisade OA, Awodele SO, Uwaidem YI. Insect pest profile of leaf amaranth (Amaranthus hybridus L) and prevention herbivory using oil-based extracts of Alium sativum L, Xylopia aethiopica Dunal and Eucalyptus globolus L. International Journal of Plant & Soil Science 2019; 28(6): 1–9. doi: 10.9734/IJPSS/2019/v28i630130
- Borisade OA, Uwaidem YI, Salami AE. Preliminary report on Fusarium oxysporum f. sp. lycopersici (Sensu lato) from some tomato producing agroecological areas in Southwestern Nigeria and susceptibility of F1-resistant tomato hybrid (F1-Lindo) to infection. Annual Research & Review in Biology 2017; 18(2): 1–9. doi: 10.9734/arrb/2017/34626
- Choi YW, Hyde KD, Ho WWH. Single spore isolation of fungi. Fungal Diversity 1999; 3: 29–38.
- Zhang CQ, Liu YH, Wu HM, et al. Baseline sensitivity of Pestalotiopsis microspora, which causes black spot disease on Chinese hickory (Carya cathayensis), to pyraclostrobin. Crop Protection 2012; 42: 256–259. doi: 10.1016/j.cropro.2012.07.018
- Ezeonuegbu BA, Abdullahi MD, Whong CMZ, et al. Characterization and phylogeny of fungi isolated from industrial wastewater using multiple genes. Scientific Reports 2022; 12(1): 2094. doi: 10.1038/s41598-022-05820-9
- Chala A, Getahun M, Alemayehu S, Tadesse M. Survey of mango anthracnose in southern Ethiopia and in-vitro screening of some essential oils against Colletotrichum gloeosporioides. International Journal of Fruit Science 2014; 14(2): 157–173.
- Afsah‐Hejri L, Jinap S, Hajeb P, et al. A review on mycotoxins in food and feed: Malaysia case study. Comprehensive Reviews in Food Science and Food Safety 2013; 12(6): 629–651. doi: 10.1111/1541-4337.12029
- Borisade OA, Oso AA, Falade MJ. Interactions of some registered agrochemicals in Nigerian farming systems with entomopathogenic fungi, Metarhizium anisopliae and Isaria farinose. Ife Journal of Science 2016; 18(4): 949–961. doi: 10.1111/1541-4337.12029
- Ladaniya MS. Commercial fresh citrus cultivars and producing countries. In: Citrus Fruit: Biology, Technology and Evaluation. Academic Press; 2008. pp. 13–65.
- Mnayer D, Fabiano-Tixier AS, Petitcolas E, et al. Chemical composition, antibacterial and antioxidant activities of six essentials oils from the Alliaceae family. Molecules 2014; 19(12): 20034–20053. doi: 10.3390/molecules191220034
- Satyal P, Craft JD, Dosoky NS, Setzer WN. The chemical compositions of the volatile oils of garlic (Allium sativum) and wild garlic (Allium vineale). Foods 2017; 6(8): 63. doi: 10.3390/foods6080063
Supporting Agencies
Copyright (c) 2023 Okon Godwin Okon, Uwaidem Yakubu Ismaila, Ukponobong Efiong Antia, Muhammad Saqlain Zaheer, Hafiz Haider Ali, Abdelhak Rhouma
License URL: https://creativecommons.org/licenses/by/4.0/

This site is licensed under a Creative Commons Attribution 4.0 International License (CC BY 4.0).

Prof. Zhengjun Qiu
Zhejiang University, China

Cheng Sun
Academician of World Academy of Productivity Science; Executive Chairman, World Confederation of Productivity Science China Chapter, China
Indexing & Archiving
In the realm of modern agriculture, the integration of cutting-edge technologies is revolutionizing the way we approach sustainable farming practices. A recent study published in Advances in Modern Agriculture titled "Classification of cotton water stress using convolutional neural networks and UAV-based RGB imagery" has garnered significant attention for its innovative approach to precision irrigation management. Conducted by researchers from Institute of Data Science and the AgriLife Research and Extension Center of Texas A&M University (authors's information is below). This study introduces a novel method for classifying cotton water stress using unmanned aerial vehicles (UAVs) and convolutional neural networks (CNNs), offering a powerful solution for optimizing water use in agriculture.
Modern agricultural technology is evolving rapidly, with scientists collaborating with leading agricultural enterprises to develop intelligent management practices. These practices utilize advanced systems that provide tailored fertilization and treatment options for large-scale land management.
This journal values human initiative and intelligence, and the employment of AI technologies to write papers that replace the human mind is expressly prohibited. When there is a suspicious submission that uses AI tools to quickly piece together and generate research results, the editorial board of the journal will reject the article, and all journals under the publisher's umbrella will prohibit all authors from submitting their articles.
Readers and authors are asked to exercise caution and strictly adhere to the journal's policy regarding the usage of Artificial Intelligence Generated Content (AIGC) tools.
Asia Pacific Academy of Science Pte. Ltd. (APACSCI) specializes in international journal publishing. APACSCI adopts the open access publishing model and provides an important communication bridge for academic groups whose interest fields include engineering, technology, medicine, computer, mathematics, agriculture and forestry, and environment.



.jpg)
.jpg)

.jpg)
