
Asia Pacific Academy of Science Pte. Ltd. (APACSCI) specializes in international journal publishing. APACSCI adopts the open access publishing model and provides an important communication bridge for academic groups whose interest fields include engineering, technology, medicine, computer, mathematics, agriculture and forestry, and environment.
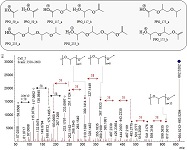
The (partial) replacement of synthetic polymers with bioplastics is due to increased production of conventional packaging plastics causing for severe environmental pollution with plastics waste. The bioplastics, however, represent complex mixtures of known and unknown (bio)polymers, fillers, plasticizers, stabilizers, flame retardant, pigments, antioxidants, hydrophobic polymers such as poly(lactic acid), polyethylene, polyesters, glycol, or poly(butylene succinate), and little is known of their chemical safety for both the environment and the human health. Polymerization reactions of bioplastics can produce no intentionally added chemicals to the bulk material, which could be toxic, as well. When polymers are used to food packing, then the latter chemicals could also migrate from the polymer to food. This fact compromises the safety for consumers, as well. The scarce data on chemical safety of bioplastics makes a gap in knowledge of their toxicity to humans and environment. Thus, development of exact analytical protocols for determining chemicals of bioplastics in environmental and food samples as well as packing polymers can only provide warrant for reliable conclusive evidence of their safety for both the human health and the environment. The task is compulsory according to legislation Directives valid to environmental protection, food control, and assessment of the risk to human health. The quantitative and structural determination of analytes is primary research task of analysis of polymers. The methods of mass spectrometry are fruitfully used for these purposes. Methodological development of exact analytical mass spectrometric tools for reliable structural analysis of bioplastics only guarantees their safety, efficacy, and quality to both humans and environment. This study, first, highlights innovative stochastic dynamics equations processing exactly mass spectrometric measurands and, thus, producing exact analyte quantification and 3D molecular and electronic structural analyses. There are determined synthetic polymers such as poly(ethylenglycol), poly(propylene glycol), and polyisoprene as well as biopolymers in bags for foodstuffs made from renewable cellulose and starch, and containing, in total within the 20,416–17,495 chemicals per sample of the composite biopolymers. Advantages of complementary employment in mass spectrometric methods and Fourier transform infrared spectroscopy is highlighted. The study utilizes ultra-high resolution electrospray ionization mass spectrometric and Fourier transform infrared spectroscopic data on biodegradable plastics bags for foodstuffs; high accuracy quantum chemical static methods, molecular dynamics; and chemometrics. There is achieved method performance |r| = 0.99981 determining poly(propylene glycol) in bag for foodstuff containing 20,416 species and using stochastic dynamics mass spectrometric formulas. The results highlight their great capability and applicability to the analytical science as well as relevance to both the fundamental research and to the industry.
Investigation of the use of Botswana coal fly ash as a material for the adsorption of arsenic from fortified water
Vol 5, Issue 1, 2024
Download PDF
Abstract
The main objective of the study was to characterize raw (RFA), water-washed (WFA) and iron-modified (Fe-WFA) Botswana coal fly ash to determine the physical and chemical properties as well as investigate its potential use as adsorbents for the removal of arsenic(III) ((As(III)) from fortified water. Scanning electron microscopy with an energy dispersive spectrometer (SEM-EDS) showed particles with irregular size and shape for all the materials and porous iron oxide flakes for Fe-WFA. The SEM-EDS, X-ray diffraction analysis (XRD) and X-ray photoelectron spectroscopy (XPS) showed the main constituents of RFA, WFA and Fe-WFA to be SiO2, Fe2O3, Al2O3 and CaO. The XPS further showed the surface composition of Fe-WFA with higher Fe content at 19.7% compared to 0.8% and 1.2% for RFA and WFA respectively. The XRF and XRD results confirmed the successful modification of WFA with iron by showing the Fe2O3 composition increasing from 12.6% of WFA to 25.5% for Fe-WFA. The inductively coupled plasma-mass spectrometry (ICP-MS) results showed continuous reduction of metal concentrations for WFA and Fe-WFA from the first to the sixth wash. The adsorption of As(III) on the adsorbents followed the Freundlich adsorption model. The maximum adsorption capacities of 0.85, 0.02 and 2.26 mgg−1 were obtained for RFA, WFA and Fe-WFA respectively.
Keywords
References
- Yao ZT. A comprehensive review on the applications of coal fly ash. Earth Science. 2014; 141: 105-121.
- Sahu BK. Improvement in California Bearing Ratio of Various Soils in Botswana by Fly Ash. Int. Ash Util. Symp. 2001; 358: 22-24.
- Xiyili H, Çetintaş S, Bingöl D. Removal of some heavy metals onto mechanically activated fly ash: Modeling approach for optimization, isotherms, kinetics and thermodynamics. Process Safety and Environmental Protection. 2017; 109: 288-300. doi: 10.1016/j.psep.2017.04.012
- Belviso C. State-of-the-art applications of fly ash from coal and biomass: A focus on zeolite synthesis processes and issues. Progress in Energy and Combustion Science. 2018; 65: 109-135. doi: 10.1016/j.pecs.2017.10.004
- Kim HJ, Pant HR, Choi NJ, et al. Composite electrospun fly ash/polyurethane fibers for absorption of volatile organic compounds from air. Chemical Engineering Journal. 2013; 230: 244-250.
- Hambira WL. Natural resources accounting: A tool for water resources management in Botswana. Physics and Chemistry of the Earth, Parts A/B/C. 2007; 32(15-18): 1310-1314.
- Huntsman-Mapila P, Mapila T, Letshwenyo M, et al. Characterization of arsenic occurrence in the water and sediments of the Okavango Delta, NW Botswana. Applied Geochemistry. 2006; 21(8): 1376-1391. doi: 10.1016/j.apgeochem.2006.05.003
- Hashim MA, Mukhopadhyay S, Sahu JN, et al. Remediation technologies for heavy metal contaminated groundwater. Journal of Environmental Management. 2011; 92(10): 2355-2388. doi: 10.1016/j.jenvman.2011.06.009
- Kobielska PA, Howarth AJ, Farha OK, et al. Metal-organic frameworks for heavy metal removal from water. Coordination Chemistry Reviews. 2018; 358: 92-107. doi: 10.1016/j.ccr.2017.12.010
- Minatel BC, Sage AP, Anderson C, et al. Environmental arsenic exposure: From genetic susceptibility to pathogenesis. Environment International. 2018; 112: 183-197. doi: 10.1016/j.envint.2017.12.017
- WHO. WHO/SDE/WSH/03.04/75 Arsenic in Drinking-water Background document for development of WHO guidelines for drinking water quality. WHO; 2011.
- Yadav LS, Mishra BK, Kumar A, et al. Arsenic removal using bagasse fly ash-iron coated and sponge iron char. Journal of Environmental Chemical Engineering. 2014; 2(3): 1467-1473.
- Zhou H, Bhattarai R, Li Y, et al. Utilization of coal fly and bottom ash pellet for phosphorus adsorption: Sustainable management and evaluation. Resources, Conservation and Recycling. 2019; 149: 372-380. doi: 10.1016/j.resconrec.2019.06.017
- Chen M, Liu Y, Zhang D, et al. Simultaneous reduction of available arsenic and cadmium in contaminated soil by iron-modified fly ash. Chemistry and Ecology. 2022; 38(7): 602-616.
- Ferrarini SF, Cardoso AM, Paprocki A, et al. Integrated Synthesis of Zeolites Using Coal Fly Ash: Element Distribution in the Products, Washing Waters and Effluent. Journal of the Brazilian Chemical Society. 2016.
- Mulder E. Pre-treatment of MSWI fly ash for useful applications. Waste Management. 1996; 16: 181-184.
- Praipipat P, Jangkorn S, Ngamsurach P. Powdered and beaded zeolite A from recycled coal fly ash with modified iron (III) oxide-hydroxide for lead adsorptions. Environmental Nanotechnology, Monitoring & Management. 2023; 20.
- Li Y, Zhang FS, Xiu FR. Arsenic (V) removal from aqueous system using adsorbent developed from a high iron-containing fly ash. Science of The Total Environment. 2009; 407(21): 5780-5786. doi: 10.1016/j.scitotenv.2009.07.017
- Li Z, Wang L, Meng J, et al. Zeolite-supported nanoscale zero-valent iron: New findings on simultaneous adsorption of Cd(II), Pb(II), and As(III) in aqueous solution and soil. Journal of Hazardous Materials. 2018; 344: 1-11. doi: 10.1016/j.jhazmat.2017.09.036
- Fu B, Hower JC, Dai S, et al. Determination of Chemical Speciation of Arsenic and Selenium in High-As Coal Combustion Ash by X-ray Photoelectron Spectroscopy: Examples from a Kentucky Stoker Ash. ACS Omega. 2018; 3(12): 17637-17645. doi: 10.1021/acsomega.8b02929
- Silva L, Ward C, Hower J, et al. Mineralogy and Leaching Characteristics of Coal Ash from a Major Brazilian Power Plant. Coal Combustion and Gasification Products. 2010; 2(1): 51-65. doi: 10.4177/ccgp-d-10-00005.1
- Madan S, Thapa U, Tiwari S, et al. Designing of a nanoscale zerovalent iron@fly ash composite as efficient and sustainable adsorbents for hexavalent chromium (Cr(VI)) from water. Environmental Science and Pollution Research. 2021; 28(18): 22474-22487. doi: 10.1007/s11356-020-11692-1
- Ochedi FO, Liu Y, Hussain A. A review on coal fly ash-based adsorbents for mercury and arsenic removal. Journal of Cleaner Production. 2020; 267: 122143. doi: 10.1016/j.jclepro.2020.122143
Supporting Agencies
Copyright (c) 2024 Kediemetse Mothibedi, Samuel Chigome, Ntebogeng Mokgalaka-Fleischmann, James Darkwa
License URL: https://creativecommons.org/licenses/by/4.0/

This site is licensed under a Creative Commons Attribution 4.0 International License (CC BY 4.0).
.jpg)
Beijing University of Technology, China



