
Asia Pacific Academy of Science Pte. Ltd. (APACSCI) specializes in international journal publishing. APACSCI adopts the open access publishing model and provides an important communication bridge for academic groups whose interest fields include engineering, technology, medicine, computer, mathematics, agriculture and forestry, and environment.
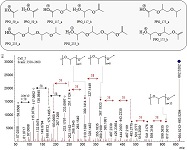
The (partial) replacement of synthetic polymers with bioplastics is due to increased production of conventional packaging plastics causing for severe environmental pollution with plastics waste. The bioplastics, however, represent complex mixtures of known and unknown (bio)polymers, fillers, plasticizers, stabilizers, flame retardant, pigments, antioxidants, hydrophobic polymers such as poly(lactic acid), polyethylene, polyesters, glycol, or poly(butylene succinate), and little is known of their chemical safety for both the environment and the human health. Polymerization reactions of bioplastics can produce no intentionally added chemicals to the bulk material, which could be toxic, as well. When polymers are used to food packing, then the latter chemicals could also migrate from the polymer to food. This fact compromises the safety for consumers, as well. The scarce data on chemical safety of bioplastics makes a gap in knowledge of their toxicity to humans and environment. Thus, development of exact analytical protocols for determining chemicals of bioplastics in environmental and food samples as well as packing polymers can only provide warrant for reliable conclusive evidence of their safety for both the human health and the environment. The task is compulsory according to legislation Directives valid to environmental protection, food control, and assessment of the risk to human health. The quantitative and structural determination of analytes is primary research task of analysis of polymers. The methods of mass spectrometry are fruitfully used for these purposes. Methodological development of exact analytical mass spectrometric tools for reliable structural analysis of bioplastics only guarantees their safety, efficacy, and quality to both humans and environment. This study, first, highlights innovative stochastic dynamics equations processing exactly mass spectrometric measurands and, thus, producing exact analyte quantification and 3D molecular and electronic structural analyses. There are determined synthetic polymers such as poly(ethylenglycol), poly(propylene glycol), and polyisoprene as well as biopolymers in bags for foodstuffs made from renewable cellulose and starch, and containing, in total within the 20,416–17,495 chemicals per sample of the composite biopolymers. Advantages of complementary employment in mass spectrometric methods and Fourier transform infrared spectroscopy is highlighted. The study utilizes ultra-high resolution electrospray ionization mass spectrometric and Fourier transform infrared spectroscopic data on biodegradable plastics bags for foodstuffs; high accuracy quantum chemical static methods, molecular dynamics; and chemometrics. There is achieved method performance |r| = 0.99981 determining poly(propylene glycol) in bag for foodstuff containing 20,416 species and using stochastic dynamics mass spectrometric formulas. The results highlight their great capability and applicability to the analytical science as well as relevance to both the fundamental research and to the industry.
Investigation of the removal of physicochemical pollutants in the Kızılırmak River by aluminum sulfate and iron sulfate coagulants
Vol 6, Issue 2, 2025
Download PDF
Abstract
Keywords
References
- Tomaszewska M, Mozia S, Morawski AW. Removal of organic matter by coagulation enhanced with adsorption on PAC. Desalination. 2004; 161(1): 79-87. doi: 10.1016/S0011-9164(04)90042-2
- Vicente C, Silva JR, Santos AD, et al. Combined Electrocoagulation and Physicochemical Treatment of Cork Boiling Wastewater. Sustainability. 2022; 14(7): 3727. doi: 10.3390/su14073727
- Benalia A, Derbal K, Amrouci Z, et al. Application of Plant-Based Coagulants and Their Mechanisms in Water Treatment: A Review. Journal of Renewable Materials. 2024; 12(4): 667-698. doi: 10.32604/jrm.2024.048306
- Gregory J, Duan J. Hydrolyzing metal salts as coagulants. Pure and Applied Chemistry. 2001; 73(12): 2017-2026. doi: 10.1351/pac200173122017
- Rizzo L, Di Gennaro A, Gallo M, et al. Coagulation/chlorination of surface water: A comparison between chitosan and metal salts. Separation and Purification Technology. 2008; 62(1): 79-85. doi: 10.1016/j.seppur.2007.12.020
- Yang Z, Gao B, Yue Q. Coagulation performance and residual aluminum speciation of Al2(SO4)3 and polyaluminum chloride (PAC) in Yellow River water treatment. Chemical Engineering Journal. 2010; 165(1): 122-132. doi: 10.1016/j.cej.2010.08.076
- Singh NK, Pandey S, Singh S, et al. Post treatment of UASB effluent by using inorganic coagulants: Role of zeta potential and characterization of solid residue. Journal of Environmental Chemical Engineering. 2016; 4(2): 1495-1503. doi: 10.1016/j.jece.2016.02.020
- Matilainen A, Lindqvist N, Tuhkanen T. Comparison of the Effiency of Aluminium and Ferric Sulphate in the Removal of Natural Organic Matter During Drinking Water Treatment Process. Environmental Technology. 2005; 26(8): 867-876. doi: 10.1080/09593332608618502
- Zhao J, Li B, Wang A, et al. Floc formation and growth mechanism during magnesium hydroxide and polyacrylamide coagulation process for reactive orange removal. Environmental Technology; 2022.
- Padilla JRR, Andrade ET, Araújo BLO, and Rios PdA. Moringa oleifera L. Cake as a potential substitute for aluminum sulphate coagulant for the treatment of surface water. Theoretical and Applied Engineering; 2023.
- Benavides CHS, Diaz JJF, Mercado T. Phytotoxicity of Natural and Synthetic Coagulants by Zea mays Lethality Assays in Treated Waters from the Magdalena River, Colombia. International Journal of ChemTech Research. 2018.
- Labastida-Núñez I, Velasco-Pérez M, Pablo-Reyes EP, et al. Evaluation of Wastewater Coagulation/Flocculation with Chitosan from Shrimp Exoskeletons Waste and Aluminum Sulfate. Revista Terra Latinoamericana; 2023. doi: 10.28940/terra.v41i0.1743
- APHA/AWWA/WEF. Standard Methods for the Examination of Water and Wastewater. Standard Methods; 2017.
- Aziz HA, Alias S, Adlan MN. The use of alum, ferric chloride and ferrous sulphate as coagulants in removing suspended solids, colour and COD from semi-aerobic landfill leachate at controlled pH. Waste Manage Res; 2007.
- Malik QH. Performance of alum and assorted coagulants in turbidity removal of muddy water. Applied Water Science. 2018; 8(1). doi: 10.1007/s13201-018-0662-5
- Daryabeigi A, Baghvand A, Zand AD, et al. Optimizing Coagulation Process for Low to High Turbidity Waters Using Aluminum and Iron Salts. American Journal of Environmental Sciences. 2010;6(5):442–8.
- Saritha V, Srinivas N, Srikanth Vuppala NV. Analysis and optimization of coagulation and flocculation process. Applied Water Science. 2015; 7(1): 451-460. doi: 10.1007/s13201-014-0262-y
- Mamelkina MA, Cotillas S, Lacasa E, et al. Removal of sulfate from mining waters by electrocoagulation. Separation and Purification Technology. 2017; 182: 87-93. doi: 10.1016/j.seppur.2017.03.044
- Loloei M, Alidadi H, Nekonam G, et al. Study of the coagulation process in wastewater treatment of dairy industries. International Journal of Environmental Health Engineering. 2014; 3(1): 12. doi: 10.4103/2277-9183.132684
- Amin A, Al Bazedi Gh, Abdel-Fatah MA. Experimental study and mathematical model of coagulation/sedimentation units for treatment of food processing wastewater. Ain Shams Engineering Journal. 2021; 12(1): 195-203. doi: 10.1016/j.asej.2020.08.001
- Bratby J. Coagulation and Flocculation in Water and Wastewater Treatment. Water Intelligence Online. 2016; 15(0): 9781780407500-9781780407500. doi: 10.2166/9781780407500
- Silva JR, Carvalho F, Vicente C, et al. Electrocoagulation treatment of cork boiling wastewater. Journal of Environmental Chemical Engineering. 2022; 10(3): 107750. doi: 10.1016/j.jece.2022.107750
- Das N, Rajput H, Aly Hassan A, et al. Application of Different Coagulants and Cost Evaluation for the Treatment of Oil and Gas Produced Water. Water. 2023; 15(3): 464. doi: 10.3390/w15030464
- Gzar HA, Jasim NA, Kseer KM. Electrocoagulation and Chemical Coagulation for Treatment of Al-Kut Textile Wastewater: A Comparative Study. Periodicals of Engineering and Natural Sciences; 2020.
- Febrianti N, Permana RNW, Ariani IK. Utilization of aloe vera as a biocoagulant for turbidity, total dissolved solid (TDS), and iron (Fe) removal in well water. IOP Conference Series: Earth and Environmental Science. 2024; 1312(1): 012007. doi: 10.1088/1755-1315/1312/1/012007
- Afarinandeh A, Ahmadpari H, Jahanpour A. Investigation of Chemical Coagulation and Flocculation Efficiency of Different Coagulants in Phosphorus pentoxide and Fluoride Removal from Kimia Daran Kavir Factory Wastewater. J Biochem Tech (2020) Special Issue. 2014.
- Chen J, Zhou Y, Chen J, et al. Self-hydrolyzing Submerged Flow Technology for Acid Coal Mine Drainage Treatment: Effective Removal of Iron Ions. Journal of Physics: Conference Series. 2024; 2706(1): 012093. doi: 10.1088/1742-6596/2706/1/012093
- Sun R, Zhang L, Wang X, et al. Elemental sulfur-driven sulfidogenic process under highly acidic conditions for sulfate-rich acid mine drainage treatment: Performance and microbial community analysis. Water Research. 2020; 185: 116230. doi: 10.1016/j.watres.2020.116230
- Wu Z, Zhang X, Pang J, et al. High-poly-aluminum chloride sulfate coagulants and their coagulation performances for removal of humic acid. RSC Advances. 2020; 10(12): 7155-7162. doi: 10.1039/c9ra10189f
Supporting Agencies
Copyright (c) 2025 Author(s)
License URL: https://creativecommons.org/licenses/by/4.0/

This site is licensed under a Creative Commons Attribution 4.0 International License (CC BY 4.0).
.jpg)
Beijing University of Technology, China



