
Asia Pacific Academy of Science Pte. Ltd. (APACSCI) specializes in international journal publishing. APACSCI adopts the open access publishing model and provides an important communication bridge for academic groups whose interest fields include engineering, technology, medicine, computer, mathematics, agriculture and forestry, and environment.
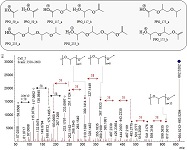
The (partial) replacement of synthetic polymers with bioplastics is due to increased production of conventional packaging plastics causing for severe environmental pollution with plastics waste. The bioplastics, however, represent complex mixtures of known and unknown (bio)polymers, fillers, plasticizers, stabilizers, flame retardant, pigments, antioxidants, hydrophobic polymers such as poly(lactic acid), polyethylene, polyesters, glycol, or poly(butylene succinate), and little is known of their chemical safety for both the environment and the human health. Polymerization reactions of bioplastics can produce no intentionally added chemicals to the bulk material, which could be toxic, as well. When polymers are used to food packing, then the latter chemicals could also migrate from the polymer to food. This fact compromises the safety for consumers, as well. The scarce data on chemical safety of bioplastics makes a gap in knowledge of their toxicity to humans and environment. Thus, development of exact analytical protocols for determining chemicals of bioplastics in environmental and food samples as well as packing polymers can only provide warrant for reliable conclusive evidence of their safety for both the human health and the environment. The task is compulsory according to legislation Directives valid to environmental protection, food control, and assessment of the risk to human health. The quantitative and structural determination of analytes is primary research task of analysis of polymers. The methods of mass spectrometry are fruitfully used for these purposes. Methodological development of exact analytical mass spectrometric tools for reliable structural analysis of bioplastics only guarantees their safety, efficacy, and quality to both humans and environment. This study, first, highlights innovative stochastic dynamics equations processing exactly mass spectrometric measurands and, thus, producing exact analyte quantification and 3D molecular and electronic structural analyses. There are determined synthetic polymers such as poly(ethylenglycol), poly(propylene glycol), and polyisoprene as well as biopolymers in bags for foodstuffs made from renewable cellulose and starch, and containing, in total within the 20,416–17,495 chemicals per sample of the composite biopolymers. Advantages of complementary employment in mass spectrometric methods and Fourier transform infrared spectroscopy is highlighted. The study utilizes ultra-high resolution electrospray ionization mass spectrometric and Fourier transform infrared spectroscopic data on biodegradable plastics bags for foodstuffs; high accuracy quantum chemical static methods, molecular dynamics; and chemometrics. There is achieved method performance |r| = 0.99981 determining poly(propylene glycol) in bag for foodstuff containing 20,416 species and using stochastic dynamics mass spectrometric formulas. The results highlight their great capability and applicability to the analytical science as well as relevance to both the fundamental research and to the industry.
Utilising mycoremediation for treatment of petroleum-contaminated soils and sediments from Ogoniland, Niger Delta, Nigeria: A kinetic insight
Vol 6, Issue 1, 2025
Download PDF
Abstract
The high prospect of mycoremediation and the lingering issues of Total Petroleum Hydrocarbons (TPHs), associated with crude oil pollution in Ogoniland, Niger Delta, Nigeria, necessitate investigations for sustainable remediation options. Therefore, mycoremediation of different contaminated soils (clayey, sandy, and loamy) and sediments collected within the study area was carried out using Pleurotus ostreatus and fermented palm wine, both optimized with Tween 80, for a period of 90 days. Results revealed variation in soils and sediments TPHs content, in the range of 120 to 525 g/kg dry soil (about 12% to 50% of TPHs contamination), which far exceeded the soil threshold value of 10 g/kg dry soil or 1%. Also, the TPHs concentrations in the soils and sediments, and the remediation efficiency were directly related to the textural properties, with the highest TPHs concentrations in clayey soils and the highest remediation efficiencies in loamy soils. Both mycoremediation agents, enhanced with Tween 80, achieved TPHs remediation above 98% in all the treatments (except in sandy soil). In all cases, the mycoremediation proceeded via pseudo second-order kinetics, and the removal rates peaked at 45–75 days. The kinetic insights also establish the initial TPHs concentration as one of the key factors influencing the remediation efficiency. This study further revealed that mycoremediation of TPHs works best in loamy soils; therefore, shifting soil properties towards those of loamy during mycoremediation is highly recommended. Consequently, with the abundant mycoremediation resources in the Niger Delta, mycoremediation can provide a sustainable option in the clean-up of the petroleum-contaminated soils and sediments.
Keywords
References
- Nwozor A. Depoliticizing environmental degradation: Revisiting the UNEP environmental assessment of Ogoniland in Nigeria’s Niger Delta region. GeoJournal. 2020; 85(3): 883–900. doi: 10.1007/s10708-019-09997-x
- Ukhurebor KE, Athar H, Adetunji CO, et al. Environmental implications of petroleum spillages in the Niger Delta region of Nigeria: A review. Journal of Environmental Management. 2021; 293: 112872. doi: 10.1016/j.jenvman.2021.112872
- Ite AE, Ibok UJ, Ite MU, Petters SW. Petroleum exploration and production: Past and present environmental issues in the Nigeria’s Niger Delta. American Journal of Environmental Protection. 2013; 1(4): 78–90. doi: 10.12691/env-1-4-2
- Uduji JI, Okolo-Obasi EN, Asongu SA. Oil extraction in Nigeria’s Ogoniland: The role of corporate social responsibility in averting a resurgence of violence. Resources Policy. 2021; 70: 101927. doi: 10.1016/j.resourpol.2020.101927
- Sam K, Zabbey N, Vincent-Akpu IF, et al. Socio-economic baseline for oil-impacted communities in Ogoniland: Towards a restoration framework in Niger Delta, Nigeria. Environmental Science and Pollution Research. 2024; 31(17): 25671–25687. doi: 10.1007/s11356-024-32805-0
- Allison C, Oriabure G, Ndimele PE, Shittu JA. Dealing with oil spill scenarios in the Niger Delta: Lessons from the past. In: The political ecology of oil and gas activities in the Nigerian aquatic ecosystem. Academic Press; 2018. pp. 351–368.
- Ben I. The development of a framework for assessing the impact of oil spills in the Ogoni land of Nigeria Niger Delta [PhD thesis]. Doctoral dissertation, Glasgow Caledonian University; 2022.
- UNEP. Environmental assessment of Ogoniland. United Nations Environment Programme. 2011.
- Nnoli NG, Olomukoro JO, Odii EC, et al. Another insight into the contamination levels at Ogoniland in Niger Delta, Nigeria, with focus on Goi Creek. Environmental Science and Pollution Research. 2021; 28(26): 34776–34792. doi: 10.1007/s11356-021-13117-z
- Anoliefo GO, Nwaokolo M, Edegbai BO, Ikhajiagbe B. Ex-situmycoremediation of petroleum polluted soils in Ogoniland, Nigeria. bioRxiv. 2023. doi: 10.1101/2023.01.08.523087
- Ite AE, Harry TA, Obadimu CO, et al. Petroleum hydrocarbons contamination of surface water and groundwater in the Niger Delta region of Nigeria. Journal of Environment Pollution and Human Health. 2018; 6(2): 51–61. doi: 10.12691/jephh-6-2-2
- Nwoma IF, Anyika VO. Restoration of the ecosystem: Ogoni cleanup and the mitigation of social tensions, 2018–2023. Human Ecology Review. 2024; 28(1): 25–40. doi: 10.22459/her.28.01.2024.03
- Ya’u A, Kurawa NS, Badamasi N. New approach for environmental restoration in Ogoniland: A proposed framework. International Journal of Intellectual Discourse. 2024; 7(1): 287–298.
- Onuh PA, Omenma TJ, Onyishi CJ, et al. Artisanal refining of crude oil in the Niger Delta: A challenge to clean-up and remediation in Ogoniland. Local Economy: The Journal of the Local Economy Policy Unit. 2021; 36(6): 468–486. doi: 10.1177/02690942211071075
- Olukaejire SJ, Ifiora CC, Osaro PA, et al. Petroleum Exploration in the Niger Delta Region and Implications for the Environment: A Review. Journal of Energy Research and Reviews. 2024; 16(5): 19–29. doi: 10.9734/jenrr/2024/v16i5350
- Ugochukwu CN, Ertel J. Negative impacts of oil exploration on biodiversity management in the Niger Delta area of Nigeria. Impact assessment and project appraisal. 2008; 26(2): 139–147.
- Mmom P, Igbuku AOO. Challenges and prospect of environmental remediation/Restoration in Niger Delta of Nigeria: The case of Ogoniland. Journal of Energy Technology and Polymers. 2015; 5: 5–5.
- Short J. Long-term effects of crude oil on developing fish: Lessons from the Exxon Valdez oil spill. Energy Sources. 2003; 25(6): 509–517. doi: 10.1080/00908310390195589
- Nriagu J, Udofia E, Ekong I, Ebuk G. Health risks associated with oil pollution in the Niger Delta, Nigeria. International Journal of Environmental Research and Public Health. 2016; 13(3): 346. doi: 10.3390/ijerph13030346
- Osuji LC, Onojake CM. Trace heavy metals associated with crude oil: A case study of Ebocha‐8 Oil‐spill‐polluted site in Niger Delta, Nigeria. Chemistry & Biodiversity. 2004; 1(11): 1708–1715.
- Dickson UJ, Udoessien EI. Physicochemical Studies of Nigeria’s Crude Oil Blends. Petroleum & Coal. 2012; 54(3).
- Kuppusamy S, Maddela NR, Megharaj M, Venkateswarlu K. Ecological impacts of total petroleum hydrocarbons. In: Total Petroleum Hydrocarbons. Springer International Publishing; 2020. pp. 95–138.
- Yang C, Wang Z, Hollebone BP, et al. Chromatographic fingerprinting analysis of crude oils and petroleum products. Handbook of Oil Spill Science and Technology. 2014; 93–163. doi: 10.1002/9781118989982.ch5
- Chinedu E, Chukwuemeka CK. Oil Spillage and Heavy Metals Toxicity Risk in the Niger Delta, Nigeria. Journal of Health and Pollution. 2018; 8(19). doi: 10.5696/2156-9614-8.19.180905
- ASTDR-Agency for Toxic Substances and Disease Registry. Toxicological Profile for Total Petroleum Hydrocarbons (Tph). U.S. Department of Health and Human Services. 1999.
- Makanjuola BC. Assessment of Air, Water Quality and Health Impact on the Environment of Petrol Stations in Ado Local Government Area of Ekiti State, Nigeria [Master’s thesis]. Kwara State University; 2019.
- Kuppusamy S, Maddela NR, Megharaj M, Venkateswarlu K. In: Total Petroleum Hydrocarbons: Environmental fate, toxicity, and remediation. Springer International Publishing; 2020. pp. 167–205.
- Bukowska B, Mokra K, Michałowicz J. Benzo[a]pyrene—Environmental Occurrence, Human Exposure, and Mechanisms of Toxicity. International Journal of Molecular Sciences. 2022; 23(11): 6348. doi: 10.3390/ijms23116348
- Nwankwoala HO, Okujagu DC. A review of wetlands and coastal resources of the Niger Delta: Potentials, Challenges and Prospects. Environment & Ecosystem Science. 2014; 5(1): 37–46. doi: 10.26480/ees.01.2021.37.46
- Adedokun OM, Kyalo M, Gnonlonfin B, et al. Mushroom: Molecular characterization of indigenous species in the Niger Delta Region of Nigeria. European Journal of Horticultural Science. 2016; 81(5): 273–280. doi: 10.17660/ejhs.2016/81.5.6
- Fubara SA, Iledare OO, Gershon O, Ejemeyovwi J. Natural resource extraction and economic performance of the Niger Delta region in Nigeria. International Journal of Energy Economics and Policy. 2019; 9(4): 188–193. doi: 10.32479/ijeep.7716
- Ezekoye CC, Amakoromo ER, Ibiene AA. Laboratory—Based Bioremediation of Hydrocarbon Polluted Mangrove Swamp Soil in the Niger Delta Using Poultry Wastes. Microbiology Research Journal International. 2017; 19(2): 1–14. doi: 10.9734/mrji/2017/15153
- McIntosh P, Schulthess CP, Kuzovkina YA, Guillard K. Bioremediation and phytoremediation of total petroleum hydrocarbons (TPH) under various conditions. International Journal of Phytoremediation. 2017; 19(8): 755–764. doi: 10.1080/15226514.2017.1284753
- Ogbonna DN, Iwegbue CMA, Sokari TG, Akoko IO. Effect of bioremediation on the growth of Okro (Abelmoshus esculetus) in the Niger Delta soils. The Environmentalist. 2007; 27(2): 303–309. doi: 10.1007/s10669-007-9006-y
- Dickson UJ, Coffey M, George Mortimer RJ, et al. Investigating the potential of sunflower species, fermented palm wine and Pleurotus ostreatus for treatment of petroleum-contaminated soil. Chemosphere. 2020; 240: 124881. doi: 10.1016/j.chemosphere.2019.124881
- AA I, OP A, UJJ I, Mt B. A critical review of oil spills in the Niger Delta aquatic environment: Causes, impacts, and bioremediation assessment. Environmental Monitoring and Assessment. 2022; 194(11). doi: 10.1007/s10661-022-10424-x
- Dickson UJ, Giadom F, Mortimer RJG, et al. Inferences from TPHs removal Kinetics during Phyto- and Myco-remediation of a Soil highly contaminated with Crude oil. 2025. unpublished work, currently under review for publication
- BS EN ISO/DIS 18400: 101-107(2016). Soil Quality Sampling; Packaging, Transport, Storage and Preservation of Samples.
- Whiting D, Card A, Wilson C, Reeder J. Estimating soil texture. Colorado State University Extension Publication. 2014.
- Salley SW, Herrick JE, Holmes CV, et al. A comparison of soil texture‐by‐feel estimates: Implications for the citizen soil scientist. Soil Science Society of America Journal. 2018; 82(6): 1526–1537. doi: 10.2136/sssaj2018.04.0137
- Yang X, Zhang Q, Li X, et al. Determination of soil texture by laser diffraction method. Soil Science Society of America Journal. 2015; 79(6): 1556–1566. doi: 10.2136/sssaj2015.04.0164
- Yang Y, Wang L, Wendroth O, et al. Is the Laser Diffraction Method Reliable for Soil Particle Size Distribution Analysis? Soil Science Society of America Journal. 2019; 83(2): 276–287. doi: 10.2136/sssaj2018.07.0252
- BSI ISO/DIS, 18400-203, (2016). Soil Quality Sampling; Packaging, Transport, Storage and Preservation of Samples.
- Adenipekun CO, Ipeaiyeda AR, Olayonwa AJ, Egbewale SO. Biodegradation of polycyclic aromatic hydrocarbons (PAHs) in spent and fresh cutting fluids contaminated soils by Pleurotus pulmonarius (Fries). Quelet and Pleurotus ostreatus (Jacq.) African Journal of Biotechnology. 2015; 14(8): 661–667. doi: 10.5897/ajb2014.14187
- Santiago-Urbina JA, Ruíz-Terán F. Microbiology and biochemistry of traditional palm wine produced around the world. International Food Research Journal. 2014; 21(4): 13–27.
- BS ISO 11464 [ (2006). ISO 11464:2006Soil quality —Pretreatment of samples for physico-chemical analysis. Available online: https://www.iso.org/standard/37718.html (accessed on 15 January 2025).
- USEPA 3546. Microwave Assisted Extraction. U.S. Environmental Protection Agency, Office of Research and Development, Washington, DC. 2007.
- Punt MM, Raghavan VGS, Belanger JMR, Pare JRJ. Microwave-assisted process (MAPTM) for the extraction of contaminants from soil. Journal of Soil Contamination. 1999; 8(5): 577–592.
- BS EN ISO 16703:2011. Soil quality. Determination of content of hydrocarbon in the range C10 to C40 by gas chromatography. Available online: https://knowledge.bsigroup.com/products/soil-quality-determination-of-content-of-hydrocarbon-in-the-range-c-sub-10-sub-to-c-sub-40-sub-by-gas-chromatography (accessed on 15 January 2025).
- Germec M, Turhan I. Predicting the experimental data of the substrate specificity of Aspergillus niger inulinase using mathematical models, estimating kinetic constants in the Michaelis—Menten equation, and sensitivity analysis. Biomass Conversion and Biorefinery. 2021; 13(12): 10641–10652. doi: 10.1007/s13399-021-01830-1
- Rytwo G, Zelkind AL. Evaluation of kinetic pseudo-order in the photocatalytic degradation of ofloxacin. Catalysts. 2021; 12(1): 24. doi: 10.3390/catal12010024
- Kajjumba GW, Emik S, Öngen A, et al. Modelling of Adsorption Kinetic Processes—Errors, Theory And Application. In: Advanced Sorption Process Applications. IntechOpen; 2018. pp. 1–19.
- Vareda JP. On validity, physical meaning, mechanism insights and regression of adsorption kinetic models. Journal of Molecular Liquids. 2023; 376: 121416. doi: 10.1016/j.molliq.2023.121416
- Azizian S. Kinetic models of sorption: A theoretical analysis. Journal of Colloid and Interface Science. 2004; 276(1): 47–52. doi: 10.1016/j.jcis.2004.03.048
- Mercado-Borrayo BM, Schouwenaars R, Litter MI, et al. Metallurgical Slag as an Efficient and Economical Adsorbent of Arsenic. Water Reclamation and Sustainability. 2014; 95–114. doi: 10.1016/b978-0-12-411645-0.00005-5
- Obradovic B. Guidelines for general adsorption kinetics modeling. Chemical Industry. 2020; 74(1): 65–70. doi: 10.2298/hemind200201006o
- Revellame ED, Fortela DL, Sharp W, et al. Adsorption kinetic modeling using pseudo-first order and pseudo-second order rate laws: A review. Cleaner Engineering and Technology. 2020; 1: 100032.
- Heredia NS, Vizuete K, Flores-Calero M, et al. Comparative statistical analysis of the release kinetics models for nanoprecipitated drug delivery systems based on poly (lactic-co-glycolic acid). PLoS One. 2022; 17(3): e0264825.
- Frost J. Regression analysis: An intuitive guide for using and interpreting linear models. Statistics By Jim Publishing; 2019.
- Huesemann MH. Guidelines for land-treating petroleum hydrocarbon-contaminated soils. Journal of Soil Contamination. 1994; 3(3): 299–318. doi: 10.1080/15320389409383471
- Rong L, Zheng X, Oba BT, et al. Activating soil microbial community using bacillus and rhamnolipid to remediate TPH contaminated soil. Chemosphere. 2021; 275: 130062. doi: 10.1016/j.chemosphere.2021.130062
- Yang ZH, Lien PJ, Huang WS, et al. Development of the risk assessment and management strategies for TPH-contaminated sites using TPH fraction methods. Journal of Hazardous, Toxic, and Radioactive Waste. 2017; 21(1): D4015003.
- Kim N, Kwon K, Park J, et al. Ex situ soil washing of highly contaminated silt loam soil using core-crosslinked amphiphilic polymer nanoparticles. Chemosphere. 2019; 224: 212–219. doi: 10.1016/j.chemosphere.2019.02.144
- Brazauskiene DM, Paulauskas V, Sabiene N. Speciation of Zn, Cu, and Pb in the soil depending on soil texture and fertilization with sewage sludge compost. Journal of Soils and Sediments. 2008; 8(3): 184–192. doi: 10.1007/s11368-008-0004-6
- Khan MAI, Biswas B, Smith E, et al. Toxicity assessment of fresh and weathered petroleum hydrocarbons in contaminated soil- a review. Chemosphere. 2018; 212: 755–767. doi: 10.1016/j.chemosphere.2018.08.094
- Palinkas LA, Downs MA, Petterson JS, Russell J. Social, cultural, and psychological impacts of the Exxon Valdez oil spill. Human Organization. 1993; 52(1): 1–13.
- Babatunde AO. Oil pollution and water conflicts in the riverine communities in Nigeria’s Niger Delta region: Challenges for and elements of problem-solving strategies. Journal of Contemporary African Studies. 2020; 38(2): 274–293. doi: 10.1080/02589001.2020.1730310
- Schapel A, Marschner P, Churchman J. Influence of clay clod size and number for organic carbon distribution in sandy soil with clay addition. Geoderma. 2019; 335: 123–132. doi: 10.1016/j.geoderma.2018.08.009
- Osman KT. Physical properties of soil. Soils: Principles, Properties and Management. Springer Netherlands; 2013. pp. 49–65.
- Khamehchiyan M, Hossein Charkhabi A, Tajik M. Effects of crude oil contamination on geotechnical properties of clayey and sandy soils. Engineering Geology. 2007; 89(3–4): 220–229. doi: 10.1016/j.enggeo.2006.10.009
- Ren X, Zeng G, Tang L, et al. Sorption, transport and biodegradation—An insight into bioavailability of persistent organic pollutants in soil. Science of The Total Environment. 2018; 610–611: 1154–1163. doi: 10.1016/j.scitotenv.2017.08.089
- Buffington JM, Montgomery DR. Effects of sediment supply on surface textures of gravel‐bed rivers. Water Resources Research. 1999; 35(11): 3523–3530. doi: 10.1029/1999wr900232
- Ferrer‐Boix C, Hassan MA. Influence of the sediment supply texture on morphological adjustments in gravel‐bed rivers. Water Resources Research. 2014; 50(11): 8868–8890. doi: 10.1002/2013wr015117
- Guyot JL, Jouanneau JM, Soares L, et al. Clay mineral composition of river sediments in the Amazon Basin. CATENA. 2007; 71(2): 340–356. doi: 10.1016/j.catena.2007.02.002
- He M, Zheng H, Huang X, et al. Yangtze River sediments from source to sink traced with clay mineralogy. Journal of Asian Earth Sciences. 2013; 69: 60–69. doi: 10.1016/j.jseaes.2012.10.001
- Al-Dhabaan FA. Mycoremediation of crude oil contaminated soil by specific fungi isolated from Dhahran in Saudi Arabia. Saudi Journal of Biological Sciences. 2021; 28(1): 73–77. doi: 10.1016/j.sjbs.2020.08.033
- Ling H, Hou J, Du M, et al. Surfactant-enhanced bioremediation of petroleum-contaminated soil and microbial community response: A field study. Chemosphere. 2023; 322: 138225. doi: 10.1016/j.chemosphere.2023.138225
- Olukanni OD, Albert AA, Farinto M, et al. Tween-80 enhanced biodegradation of naphthalene by Klebsiella quasipneumoniae. Antonie van Leeuwenhoek. 2023; 116(7): 697–709. doi: 10.1007/s10482-023-01839-8
- Sumerta IN, Ruan X, Howell K. The forgotten wine: Understanding the ecology and composition of palm wine fermentation. bioRxiv. 2024. doi: 10.1101/2024.04.26.591403
- Gul H, Akbar S, Gohar S, Mazhar F. Biochemical Analysis and Therapeutic Potential of Extract from Mushroom (Pleurotus ostreatus). International Journal of Applied And Clinical Research. 2024; 2(1): 84–100.
- Nyangwire B, Ocimati W, Tazuba AF, et al. Pleurotus ostreatus is a potential biological control agent of root-knot nematodes in eggplant (Solanum melongena). Frontiers in Agronomy. 2024; 6. doi: 10.3389/fagro.2024.1464111
- Timmerman MD, Fuller LG, Burton DL. The effects of a crude oil spill on microbiological indices of soil biological quality. Canadian Journal of Soil Science. 2003; 83(2): 173–181. doi: 10.4141/s01-039
- Franco I, Contin M, Bragato G, De Nobili M. Microbiological resilience of soils contaminated with crude oil. Geoderma. 2004; 121(1–2): 17–30. doi: 10.1016/j.geoderma.2003.10.002
- Baek KH, Yoon BD, Kim BH, et al. Monitoring of microbial diversity and activity during bioremediation of crude oil-contaminated soil with different treatments. Journal of Microbiology and Biotechnology. 2007; 17(1): 67–73.
- Gao YC, Guo SH, Wang JN, et al. Effects of different remediation treatments on crude oil contaminated saline soil. Chemosphere. 2014; 117: 486–493. doi: 10.1016/j.chemosphere.2014.08.070
- Margesin R, Hämmerle M, Tscherko D. Microbial activity and community composition during bioremediation of diesel-oil-contaminated soil: Effects of hydrocarbon concentration, fertilizers, and incubation time. Microbial Ecology. 2007; 53(2): 259–269. doi: 10.1007/s00248-006-9136-7
- Adenipekun CO, Lawal R. Uses of mushrooms in bioremediation: A review. Biotechnology and Molecular Biology Reviews. 2012; 7(3). doi: 10.5897/bmbr12.006
- Melero S, Porras JCR, Herencia JF, Madejon E. Chemical and biochemical properties in a silty loam soil under conventional and organic management. Soil and Tillage Research. 2006; 90(1–2): 162–170. doi: 10.1016/j.still.2005.08.016
- Mitchell JK, Soga K. In: Fundamentals of soil behavior, 3rd ed. John Wiley & Sons; 2005.
- Aggelides SM, Londra PA. Effects of compost produced from town wastes and sewage sludge on the physical properties of a loamy and a clay soil. Bioresource technology. 2000; 71(3): 253–259.
- Yu H, Ding W, Luo J, et al. Long-term application of organic manure and mineral fertilizers on aggregation and aggregate-associated carbon in a sandy loam soil. Soil and Tillage Research. 2012; 124: 170–177. doi: 10.1016/j.still.2012.06.011
- Hamza MA, Anderson WK. Responses of soil properties and grain yields to deep ripping and gypsum application in a compacted loamy sand soil contrasted with a sandy clay loam soil in Western Australia. Australian Journal of Agricultural Research. 2003; 54(3): 273–282. doi: 10.1071/ar02102
- Bojes HK, Pope PG. Characterization of EPA’s 16 priority pollutant polycyclic aromatic hydrocarbons (PAHs) in tank bottom solids and associated contaminated soils at oil exploration and production sites in Texas. Regulatory Toxicology and Pharmacology. 2007; 47(3): 288–295. doi: 10.1016/j.yrtph.2006.11.007
- HO YS. Review of second-order models for adsorption systems. Journal of Hazardous Materials. 2006; 136(3): 681–689. doi: 10.1016/j.jhazmat.2005.12.043
Supporting Agencies
Copyright (c) 2025 Author(s)
License URL: https://creativecommons.org/licenses/by/4.0/

This site is licensed under a Creative Commons Attribution 4.0 International License (CC BY 4.0).
.jpg)
Beijing University of Technology, China



