
Asia Pacific Academy of Science Pte. Ltd. (APACSCI) specializes in international journal publishing. APACSCI adopts the open access publishing model and provides an important communication bridge for academic groups whose interest fields include engineering, technology, medicine, computer, mathematics, agriculture and forestry, and environment.
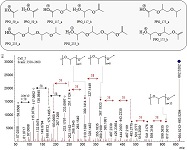
The (partial) replacement of synthetic polymers with bioplastics is due to increased production of conventional packaging plastics causing for severe environmental pollution with plastics waste. The bioplastics, however, represent complex mixtures of known and unknown (bio)polymers, fillers, plasticizers, stabilizers, flame retardant, pigments, antioxidants, hydrophobic polymers such as poly(lactic acid), polyethylene, polyesters, glycol, or poly(butylene succinate), and little is known of their chemical safety for both the environment and the human health. Polymerization reactions of bioplastics can produce no intentionally added chemicals to the bulk material, which could be toxic, as well. When polymers are used to food packing, then the latter chemicals could also migrate from the polymer to food. This fact compromises the safety for consumers, as well. The scarce data on chemical safety of bioplastics makes a gap in knowledge of their toxicity to humans and environment. Thus, development of exact analytical protocols for determining chemicals of bioplastics in environmental and food samples as well as packing polymers can only provide warrant for reliable conclusive evidence of their safety for both the human health and the environment. The task is compulsory according to legislation Directives valid to environmental protection, food control, and assessment of the risk to human health. The quantitative and structural determination of analytes is primary research task of analysis of polymers. The methods of mass spectrometry are fruitfully used for these purposes. Methodological development of exact analytical mass spectrometric tools for reliable structural analysis of bioplastics only guarantees their safety, efficacy, and quality to both humans and environment. This study, first, highlights innovative stochastic dynamics equations processing exactly mass spectrometric measurands and, thus, producing exact analyte quantification and 3D molecular and electronic structural analyses. There are determined synthetic polymers such as poly(ethylenglycol), poly(propylene glycol), and polyisoprene as well as biopolymers in bags for foodstuffs made from renewable cellulose and starch, and containing, in total within the 20,416–17,495 chemicals per sample of the composite biopolymers. Advantages of complementary employment in mass spectrometric methods and Fourier transform infrared spectroscopy is highlighted. The study utilizes ultra-high resolution electrospray ionization mass spectrometric and Fourier transform infrared spectroscopic data on biodegradable plastics bags for foodstuffs; high accuracy quantum chemical static methods, molecular dynamics; and chemometrics. There is achieved method performance |r| = 0.99981 determining poly(propylene glycol) in bag for foodstuff containing 20,416 species and using stochastic dynamics mass spectrometric formulas. The results highlight their great capability and applicability to the analytical science as well as relevance to both the fundamental research and to the industry.
Addressing wastewater treatment challenges in developing nations: A standardized framework for sustainable adsorption techniques in small and medium industries
Vol 6, Issue 1, 2025
Download PDF
Abstract
Water pollution has become a major challenge for many low-income and developing countries, leading to a shortage of clean water for daily activities. The review section of this study merges findings from different studies on wastewater treatment, which explored various techniques categorized primarily into physical, biological, and chemical methods. Among these, adsorption—a physical method was identified as the most cost-effective and environmentally friendly approach, primarily because the materials needed for it are widely available in nature. A major gap observed in all the studies reviewed was the lack of the application of the adsorption technique on an industrial scale, which stems mainly from the absence of standardization, as the study reveals. To bridge this gap, we develop a standardized framework for adsorption techniques in small and medium industries with clear guidelines on how to implement adsorption-based wastewater treatment. It incorporates sustainable practices, climate change considerations, and water risk management to ensure long-term environmental and economic benefits.
Keywords
References
- World Health Organization. Drinking-water. World Health Organization; 2019.
- World Health Organization. Almost 2 billion people depend on health care facilities without basic water services. World Health Organization; 2020.
- Dakkak A. Water pollution worries in developing world. Available online: https://www.ecomena.org/water-pollution/ (accessed on 2 June 2024).
- Ali I, Asim M, Khan TA. Low cost adsorbents for the removal of organic pollutants from wastewater. Journal of Environmental Management. 2012; 113: 170–183.
- Nishat A, Yusuf M, Qadir A, et al. Wastewater treatment: A short assessment on available techniques. Alexandria Engineering Journal. 2023; 76: 505–516.
- Rashid R, Shafiq I, Akhter P, et al. A state-of-the-art review on wastewater treatment techniques: The effectiveness of adsorption method. Environmental Science and Pollution Research. 2021; 28: 9050–9066
- Tsoutsa EK, Tolkou AK, Kyzas GZ, et al. An update on agricultural wastes used as natural adsorbents or coagulants in single or combined systems for the removal of dyes from wastewater. Water, Air and Soil Pollution. 2024; 235(3).
- Ngeno EC, Mbuci KE, Necibi MC, et al. Sustainable re-utilization of waste materials as adsorbents for water and wastewater treatment in Africa: Recent studies, research gaps, and way forward for emerging economies. Environmental Advances. 2022; 9: 100282.
- Bekchanov D, Mukhamediev M, Yarmanov S, et al. Functionalizing natural polymers to develop green adsorbents for wastewater treatment applications. Carbohydrate Polymers. 2024; 323: 121397.
- Wang T, Jiang M, Yu X, et al. Application of lignin adsorbent in wastewater treatment: A review. Separation and Purification Technology. 2022; 302: 122116.
- Kabir MM, Mouna SSP, Akter S, et al. Tea waste based natural adsorbent for toxic pollutant removal from waste samples. Journal of Molecular Liquids. 2021; 322.
- Abdulredha M, Kadhim NR, Hussein AH, et al. Zeolite as a natural adsorbent for nitrogenous compounds being removed from water. In: Proceedings of the 4th International Conference on Engineering Sciences (ICES 2020); 5–6 December 2020; Kerbala, Iraq.
- Dlamini DS, Tesha JM, Vilakati GD, et al. A critical review of selected membrane- and powder-based adsorbents for water treatment: Sustainability and effectiveness. Journal of Cleaner Production. 2020; 277: 123497.
- Maheshwari U, Thakur RV, Deshpande D, et al. Efficiency evaluation of orange and banana peels for dye removal from synthetic industrial effluent. Materials-Today-Proceedings. 2022. doi: 10.1016/j. matpr.2022.11.023
- Banerjee SK, Majumdar S, Dutta AC, et al. Active carbon from coconut shell. Indian Journal of Technology. 1976; 14: 45–49.
- Hitchcock SJ, McEnaney B, Watling SJ. Fibrous active carbons from coir. Journal of Chemical Technology Biotechnology. 1983; 33(3): 157–163.
- Lucchesi A, Maschio G. Semi-active carbon and aromatics produced from the pyrolysis of scrap tyres. Conservation and Recycling. 1983; 6(3): 85–90.
- Ogassawara S, Kuroda SM, Wakao N. Preparation of activated carbon by thermal decomposition of used automotive tyres. Industrial and Engineering Chemical Research. 1987; 26(12): 2552–2556.
- Paprowicz JT. Activated carbons for phenols removal from wastewaters. Environmental Technology. 1990; 11(1): 71–81.
- Guedes deCarvalho RA, Gonzalez-Beca CG, Sampaio MN, et al. Use of pine bark for preparation of activated carbon and as a soil conditioner. Agricultural Wastes.1984; 9(3): 231–238.
- Edgehill RU, Lu GQ. Adsorption characteristics of carbonized bark for phenol and pentachlorophenol. Journal of Chemical Technology Biotechnology. 1998; 71(1): 27–37.
- Asfour HM, Fadali OA, Nasser MM, et al. Equilibrium studies on adsorption of basic dyes on hardwood. Journal of Chemical Technology Biotechnology. 1985; 35(1): 21–27.
- Youseff AM, Mostafa MR, Dorgham EM. Surface properties and decolorizing power of carbons from rice husks. Afinidad. 1990; 425: 41–44.
- El-Guendi MS. Adsorption kinetics of cationic dyestuffs on to natural clay. Adsorption Science and Technology. 1996; 13: 295–303.
- Dong Y, Wu D, Chen X, et al. Adsorption of bisphenol A from water by surfactant-modified zeolite. Journal of Colloid and Interface Science. 2010; 348(2): 585–590.
- Chinedu N. Removal of red dye and phenol from simulated waste water by adsorption using activated carbon produced from rubber seed husk [Bachelor’s thesis]. Nnamdi Azikiwe University; 2021.
Supporting Agencies
Copyright (c) 2025 Author(s)
License URL: https://creativecommons.org/licenses/by/4.0/

This site is licensed under a Creative Commons Attribution 4.0 International License (CC BY 4.0).
.jpg)
Beijing University of Technology, China



