
Asia Pacific Academy of Science Pte. Ltd. (APACSCI) specializes in international journal publishing. APACSCI adopts the open access publishing model and provides an important communication bridge for academic groups whose interest fields include engineering, technology, medicine, computer, mathematics, agriculture and forestry, and environment.
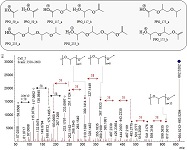
The (partial) replacement of synthetic polymers with bioplastics is due to increased production of conventional packaging plastics causing for severe environmental pollution with plastics waste. The bioplastics, however, represent complex mixtures of known and unknown (bio)polymers, fillers, plasticizers, stabilizers, flame retardant, pigments, antioxidants, hydrophobic polymers such as poly(lactic acid), polyethylene, polyesters, glycol, or poly(butylene succinate), and little is known of their chemical safety for both the environment and the human health. Polymerization reactions of bioplastics can produce no intentionally added chemicals to the bulk material, which could be toxic, as well. When polymers are used to food packing, then the latter chemicals could also migrate from the polymer to food. This fact compromises the safety for consumers, as well. The scarce data on chemical safety of bioplastics makes a gap in knowledge of their toxicity to humans and environment. Thus, development of exact analytical protocols for determining chemicals of bioplastics in environmental and food samples as well as packing polymers can only provide warrant for reliable conclusive evidence of their safety for both the human health and the environment. The task is compulsory according to legislation Directives valid to environmental protection, food control, and assessment of the risk to human health. The quantitative and structural determination of analytes is primary research task of analysis of polymers. The methods of mass spectrometry are fruitfully used for these purposes. Methodological development of exact analytical mass spectrometric tools for reliable structural analysis of bioplastics only guarantees their safety, efficacy, and quality to both humans and environment. This study, first, highlights innovative stochastic dynamics equations processing exactly mass spectrometric measurands and, thus, producing exact analyte quantification and 3D molecular and electronic structural analyses. There are determined synthetic polymers such as poly(ethylenglycol), poly(propylene glycol), and polyisoprene as well as biopolymers in bags for foodstuffs made from renewable cellulose and starch, and containing, in total within the 20,416–17,495 chemicals per sample of the composite biopolymers. Advantages of complementary employment in mass spectrometric methods and Fourier transform infrared spectroscopy is highlighted. The study utilizes ultra-high resolution electrospray ionization mass spectrometric and Fourier transform infrared spectroscopic data on biodegradable plastics bags for foodstuffs; high accuracy quantum chemical static methods, molecular dynamics; and chemometrics. There is achieved method performance |r| = 0.99981 determining poly(propylene glycol) in bag for foodstuff containing 20,416 species and using stochastic dynamics mass spectrometric formulas. The results highlight their great capability and applicability to the analytical science as well as relevance to both the fundamental research and to the industry.
Preparation of biochar-based composites and their use in the remediation of environments contaminated with organic pollutants
Vol 4, Issue 2, 2023
Download PDF
Abstract
Biochar offers several advantages, including high carbon content, a large specific surface area, a well-developed pore structure, and excellent adsorption capabilities. It is extensively utilized in improving soil quality, remediating environmental pollution, and contributing to carbon sequestration and emission reduction. However, practical challenges such as the low bulk density of biochar, difficulties in solid-liquid separation, and the need to enhance its long-term effectiveness and stability persist. To address these issues, biochar composites with improved remediation efficiency for polluted environments have been developed by combining biochar with metal materials, photocatalysts, and clay minerals, garnering significant attention. This systematic review examines the preparation methods and applications of biochar-based composite materials in environmental pollution control, analyzes the mechanisms for removing organic pollutants, and discusses future research and development trends for biochar materials, aiming to provide new insights for the creation and practical use of high-efficiency biochar composites.
Keywords
References
- Melia PM, Busquets R, Hooda PS, et al. Driving forces and barriers in the removal of phosphorus from water using crop residue, wood and sewage sludge derived biochars. Science of The Total Environment. 2019; 675: 623-631. doi: 10.1016/j.scitotenv.2019.04.232
- Chen W, Zhang W, Meng J. Research progress and Prospect of agricultural biochar. China Agricultural Science (Chinese). 2013; 46(16): 3324-3333.
- Yi Y, Huang Z, Lu B, et al. Magnetic biochar for environmental remediation: A review. Bioresource Technology. 2020; 298: 122468. doi: 10.1016/j.biortech.2019.122468
- Hu B, Ai Y, Jin J, et al. Efficient elimination of organic and inorganic pollutants by biochar and biochar-based materials. Biochar. 2020; 2(1): 47-64. doi: 10.1007/s42773-020-00044-4
- Liu S, Xu W hua, Liu Y guo, et al. Facile synthesis of Cu(II) impregnated biochar with enhanced adsorption activity for the removal of doxycycline hydrochloride from water. Science of The Total Environment. 2017; 592: 546-553. doi: 10.1016/j.scitotenv.2017.03.087
- Ghodake GS, Shinde SK, Kadam AA, et al. Review on biomass feedstocks, pyrolysis mechanism and physicochemical properties of biochar: State-of-the-art framework to speed up vision of circular bioeconomy. Journal of Cleaner Production. 2021; 297: 126645. doi: 10.1016/j.jclepro.2021.126645
- Fang J, Jin L, Cheng L, et al. Research Progress on the stability of biochar in environment (Chinese). Journal of soil. 2019; 56(5): 1034-1047.
- Dai Y, Zhang N, Xing C, et al. The adsorption, regeneration and engineering applications of biochar for removal organic pollutants: A review. Chemosphere. 2019; 223: 12-27. doi: 10.1016/j.chemosphere.2019.01.161
- Peng B, Chen L, Que C, et al. Adsorption of Antibiotics on Graphene and Biochar in Aqueous Solutions Induced by π-π Interactions. Scientific Reports. 2016; 6(1). doi: 10.1038/srep31920
- Zhu D, Zhou Q. Research Progress on Application of functional nano materials in remediation of heavy metal polluted water (Chinese). Journal of Agricultural Environmental Sciences. 2018; 37(8): 1551-1564.
- Keiluweit M, Nico PS, Johnson MG, et al. Dynamic Molecular Structure of Plant Biomass-Derived Black Carbon (Biochar). Environmental Science & Technology. 2010; 44(4): 1247-1253. doi: 10.1021/es9031419
- Wang S, Zhang H, Huang H, et al. Influence of temperature and residence time on characteristics of biochars derived from agricultural residues: A comprehensive evaluation. Process Safety and Environmental Protection. 2020; 139: 218-229. doi: 10.1016/j.psep.2020.03.028
- Ameloot N, Graber ER, Verheijen FGA, et al. Interactions between biochar stability and soil organisms: review and research needs. European Journal of Soil Science. 2013; 64(4): 379-390. doi: 10.1111/ejss.12064
- Feng Z, Yuan R, Wang F, et al. Preparation of magnetic biochar and its application in catalytic degradation of organic pollutants: A review. Science of The Total Environment. 2021; 765: 142673. doi: 10.1016/j.scitotenv.2020.142673
- Zhu Z, Fan W, Liu Z, et al. Fabrication of the metal-free biochar-based graphitic carbon nitride for improved 2-Mercaptobenzothiazole degradation activity. Journal of Photochemistry and Photobiology A: Chemistry. 2018; 358: 284-293. doi: 10.1016/j.jphotochem.2018.03.027
- Inyang M, Gao B, Zimmerman A, et al. Synthesis, characterization, and dye sorption ability of carbon nanotube–biochar nanocomposites. Chemical Engineering Journal. 2014; 236: 39-46. doi: 10.1016/j.cej.2013.09.074
- Yu Z, Huang Y, Lian F. Study on the adsorption properties of biochar manganese oxide composite for arsenic (Ⅲ) (Chinese). Journal of Agricultural Environmental Sciences. 2015; 34(1): 155-161.
- Tang Z, Zhao S, Qian Y, et al. Research Progress on the formation mechanism of persistent free radicals in biochar and its environmental application. Progress in chemical industry. 2020; 39(4): 1521-1527.
- Chakraborty S, Favre F, Banerjee D, et al. U(VI) Sorption and Reduction by Fe(II) Sorbed on Montmorillonite. Environmental Science & Technology. 2010; 44(10): 3779-3785. doi: 10.1021/es903493n
- Wang S, Zhao M, Zhou M, et al. Biochar-supported nZVI (nZVI/BC) for contaminant removal from soil and water: A critical review. Journal of Hazardous Materials. 2019; 373: 820-834. doi: 10.1016/j.jhazmat.2019.03.080
- Alagha O, Manzar MS, Zubair M, et al. Magnetic Mg-Fe/LDH Intercalated Activated Carbon Composites for Nitrate and Phosphate Removal from Wastewater: Insight into Behavior and Mechanisms. Nanomaterials. 2020; 10(7): 1361. doi: 10.3390/nano10071361
- Wu H, Feng Q, Yang H, et al. Enhanced phenanthrene removal in aqueous solution using modified biochar supported nano zero-valent iron. Environmental Technology. 2018; 40(23): 3114-3123. doi: 10.1080/09593330.2018.1549104
- Zhang Q, Wang Y, Wang Z, et al. Active biochar support nano zero-valent iron for efficient removal of U(VI) from sewage water. Journal of Alloys and Compounds. 2021; 852: 156993. doi: 10.1016/j.jallcom.2020.156993
- Fan X, Cao Q, Meng F, et al. A Fenton-like system of biochar loading Fe–Al layered double hydroxides (FeAl-LDH@BC)/H2O2 for phenol removal. Chemosphere. 2021; 266: 128992. doi: 10.1016/j.chemosphere.2020.128992
- Wang X, Maeda K, Thomas A, et al. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nature Materials. 2008; 8(1): 76-80. doi: 10.1038/nmat2317
- Ye S, Wang R, Wu MZ, et al. A review on g-C3N4 for photocatalytic water splitting and CO2 reduction. Applied Surface Science. 2015; 358: 15-27. doi: 10.1016/j.apsusc.2015.08.173
- Gui M, Li F, Wang Y, et al. Photocatalytic degradation of organic pollutants by modified graphite phase carbon nitride (Chinese). Journal of Liaoning University of petrochemical technology. 2019; 39(2): 1-9.
- Lam SM, Sin JC, Mohamed AR. A review on photocatalytic application of g-C3N4/semiconductor (CNS) nanocomposites towards the erasure of dyeing wastewater. Materials Science in Semiconductor Processing. 2016; 47: 62-84. doi: 10.1016/j.mssp.2016.02.019
- Meng L, Yin W, Wang S, et al. Photocatalytic behavior of biochar-modified carbon nitride with enriched visible-light reactivity. Chemosphere. 2020; 239: 124713. doi: 10.1016/j.chemosphere.2019.124713
- Zong L, Tang J, Mou B, et al. Research progress of attapulgite/carbon composite adsorbents (Chinese). Progress in chemical industry. 2021; 40(1): 282-296.
- Rozic M, Cerjan-Stefanovic S, Kurajica S, et al. Ammonical nitrogen removal from water by treatment with clays and zeolites. Water Research. 2000; 34(14): 3675-3681.
- Xu Y, Wu X, Liu C, et al. Effect of biomass carbon source on organic modification and adsorption properties of attapulgite (Chinese). Chemical reaction engineering and process. 2013; 29(2): 119-124.
- Liu W, Yao C, Wang M, et al. Kinetics and thermodynamics characteristics of cationic yellow X‐GL adsorption on attapulgite/rice hull‐based activated carbon nanocomposites. Environmental Progress & Sustainable Energy. 2012; 32(3): 655-662. doi: 10.1002/ep.11680
- Premarathna KSD, Rajapaksha AU, Sarkar B, et al. Biochar-based engineered composites for sorptive decontamination of water: A review. Chemical Engineering Journal. 2019; 372: 536-550. doi: 10.1016/j.cej.2019.04.097
- Pi L, Jiang R, Zhou W, et al. g-C3N4 Modified biochar as an adsorptive and photocatalytic material for decontamination of aqueous organic pollutants. Applied Surface Science. 2015; 358: 231-239. doi: 10.1016/j.apsusc.2015.08.176
- Inyang M, Gao B, Zimmerman A, et al. Sorption and cosorption of lead and sulfapyridine on carbon nanotube-modified biochars. Environmental Science and Pollution Research. 2014; 22(3): 1868-1876. doi: 10.1007/s11356-014-2740-z
- Zhang J, Lu M, Wan J, et al. Effects of pH, dissolved humic acid and Cu2+ on the adsorption of norfloxacin on montmorillonite-biochar composite derived from wheat straw. Biochemical Engineering Journal. 2018; 130: 104-112. doi: 10.1016/j.bej.2017.11.018
- Ahmed MB, Zhou JL, Ngo HH, et al. Single and competitive sorption properties and mechanism of functionalized biochar for removing sulfonamide antibiotics from water. Chemical Engineering Journal. 2017; 311: 348-358. doi: 10.1016/j.cej.2016.11.106
- Tang J, Mu B, Zong L, et al. One-step synthesis of magnetic attapulgite/carbon supported NiFe-LDHs by hydrothermal process of spent bleaching earth for pollutants removal. Journal of Cleaner Production. 2018; 172: 673-685. doi: 10.1016/j.jclepro.2017.10.181
- Tan X, Liu Y, Gu Y, et al. Biochar pyrolyzed from MgAl-layered double hydroxides pre-coated ramie biomass (Boehmeria nivea (L.) Gaud.): Characterization and application for crystal violet removal. Journal of Environmental Management. 2016; 184: 85-93. doi: 10.1016/j.jenvman.2016.08.070
- Ahmed MB, Zhou JL, Ngo HH, et al. Nano-Fe 0 immobilized onto functionalized biochar gaining excellent stability during sorption and reduction of chloramphenicol via transforming to reusable magnetic composite. Chemical Engineering Journal. 2017; 322: 571-581. doi: 10.1016/j.cej.2017.04.063
- Shi J. On the Synergetic Catalytic Effect in Heterogeneous Nanocomposite Catalysts. Chemical Reviews. 2012; 113(3): 2139-2181. doi: 10.1021/cr3002752
- He S, Xiao K, Chen XZ, et al. Enhanced photoelectrocatalytic activity of direct Z-scheme porous amorphous carbon nitride/manganese dioxide nanorod arrays. Journal of Colloid and Interface Science. 2019; 557: 644-654. doi: 10.1016/j.jcis.2019.09.035
- Li Y, Zhang P, Wang D, et al. Adsorption characteristics of Rhodamine B on magnetic Ginkgo biloba biochar (Chinese). Functional materials. 2019; 50(5): 5121-5127.
- Eltaweil AS, Ali Mohamed H, Abd El-Monaem EM, et al. Mesoporous magnetic biochar composite for enhanced adsorption of malachite green dye: Characterization, adsorption kinetics, thermodynamics and isotherms. Advanced Powder Technology. 2020; 31(3): 1253-1263. doi: 10.1016/j.apt.2020.01.005
- Mubarak NM, Kundu A, Sahu JN, et al. Synthesis of palm oil empty fruit bunch magnetic pyrolytic char impregnating with FeCl3 by microwave heating technique. Biomass and Bioenergy. 2014; 61: 265-275. doi: 10.1016/j.biombioe.2013.12.021
- Qiu Y, Xu X, Xu Z, et al. Contribution of different iron species in the iron-biochar composites to sorption and degradation of two dyes with varying properties. Chemical Engineering Journal. 2020; 389: 124471. doi: 10.1016/j.cej.2020.124471
- Jung KW, Choi BH, Jeong TU, et al. Facile synthesis of magnetic biochar/Fe3O4 nanocomposites using electro-magnetization technique and its application on the removal of acid orange 7 from aqueous media. Bioresource Technology. 2016; 220: 672-676. doi: 10.1016/j.biortech.2016.09.035
- Meili L, Lins PV, Zanta CLPS, et al. MgAl-LDH/Biochar composites for methylene blue removal by adsorption. Applied Clay Science. 2019; 168: 11-20. doi: 10.1016/j.clay.2018.10.012
- Wang H, Zhao W, Chen Y, et al. Nickel aluminum layered double oxides modified magnetic biochar from waste corncob for efficient removal of acridine orange. Bioresource Technology. 2020; 315: 123834. doi: 10.1016/j.biortech.2020.123834
- Chen S, Zhou M, Wang HF, et al. Adsorption of Reactive Brilliant Red X-3B in Aqueous Solutions on Clay–Biochar Composites from Bagasse and Natural Attapulgite. Water. 2018; 10(6): 703. doi: 10.3390/w10060703
- Yao Y, Gao B, Fang J, et al. Characterization and environmental applications of clay–biochar composites. Chemical Engineering Journal. 2014; 242: 136-143. doi: 10.1016/j.cej.2013.12.062
- Wu F, Liu W, Qiu J, et al. Enhanced photocatalytic degradation and adsorption of methylene blue via TiO2 nanocrystals supported on graphene-like bamboo charcoal. Applied Surface Science. 2015; 358: 425-435. doi: 10.1016/j.apsusc.2015.08.161
- Mian MM, Liu G, Yousaf B, et al. One-step synthesis of N-doped metal/biochar composite using NH3-ambiance pyrolysis for efficient degradation and mineralization of Methylene Blue. Journal of Environmental Sciences. 2019; 78: 29-41. doi: 10.1016/j.jes.2018.06.014
- Fazal T, Razzaq A, Javed F, et al. Integrating adsorption and photocatalysis: A cost effective strategy for textile wastewater treatment using hybrid biochar-TiO2 composite. Journal of Hazardous Materials. 2020; 390: 121623. doi: 10.1016/j.jhazmat.2019.121623
- Xu J, Hu P, Lv Ji, et al. Pollution characteristics and control measures of antibiotics and resistance genes in water environment (Chinese). Science and technology Herald. 2018; 36(15): 13-23.
- Li R, Wang Z, Zhao X, et al. Magnetic biochar-based manganese oxide composite for enhanced fluoroquinolone antibiotic removal from water. Environmental Science and Pollution Research. 2018; 25(31): 31136-31148. doi: 10.1007/s11356-018-3064-1
- Gao M, Zhang Y, Gong X, et al. Removal mechanism of di-n-butyl phthalate and oxytetracycline from aqueous solutions by nano-manganese dioxide modified biochar. Environmental Science and Pollution Research. 2017; 25(8): 7796-7807. doi: 10.1007/s11356-017-1089-5
- Mansoori S, Davarnejad R, Ozumchelouei EJ, et al. Activated biochar supported iron-copper oxide bimetallic catalyst for degradation of ciprofloxacin via photo-assisted electro-Fenton process: A mild pH condition. Journal of Water Process Engineering. 2021; 39: 101888. doi: 10.1016/j.jwpe.2020.101888
- Tan X, Liu S, Liu Y, et al. One-pot synthesis of carbon supported calcined-Mg/Al layered double hydroxides for antibiotic removal by slow pyrolysis of biomass waste. Scientific Reports. 2016; 6(1). doi: 10.1038/srep39691
- Li G, Huang Z, Chen C, et al. Simultaneous adsorption of trace sulfamethoxazole and hexavalent chromium by biochar/MgAl layered double hydroxide composites. Environmental Chemistry. 2019; 16(1): 68. doi: 10.1071/en18132
- Ashiq A, Sarkar B, Adassooriya N, et al. Sorption process of municipal solid waste biochar-montmorillonite composite for ciprofloxacin removal in aqueous media. Chemosphere. 2019; 236: 124384. doi: 10.1016/j.chemosphere.2019.124384
- Liang G, Wang Z, Yang X, et al. Efficient removal of oxytetracycline from aqueous solution using magnetic montmorillonite-biochar composite prepared by one step pyrolysis. Science of The Total Environment. 2019; 695: 133800. doi: 10.1016/j.scitotenv.2019.133800
- Ashiq A, Adassooriya NM, Sarkar B, et al. Municipal solid waste biochar-bentonite composite for the removal of antibiotic ciprofloxacin from aqueous media. Journal of Environmental Management. 2019; 236: 428-435. doi: 10.1016/j.jenvman.2019.02.006
- Premarathna KSD, Rajapaksha AU, Adassoriya N, et al. Clay-biochar composites for sorptive removal of tetracycline antibiotic in aqueous media. Journal of Environmental Management. 2019; 238: 315-322. doi: 10.1016/j.jenvman.2019.02.069
- Kim JR, Kan E. Heterogeneous photocatalytic degradation of sulfamethoxazole in water using a biochar-supported TiO2 photocatalyst. Journal of Environmental Management. 2016; 180: 94-101. doi: 10.1016/j.jenvman.2016.05.016
- Wang Y, Li F, Rong X, et al. Remediation of Petroleum-contaminated Soil Using Bulrush Straw Powder, Biochar and Nutrients. Bulletin of Environmental Contamination and Toxicology. 2017; 98(5): 690-697. doi: 10.1007/s00128-017-2064-z
- Ye S, Yan M, Tan X, et al. Facile assembled biochar-based nanocomposite with improved graphitization for efficient photocatalytic activity driven by visible light. Applied Catalysis B: Environmental. 2019; 250: 78-88. doi: 10.1016/j.apcatb.2019.03.004
- Xiao Y, Lyu H, Yang C, et al. Graphitic carbon nitride/biochar composite synthesized by a facile ball-milling method for the adsorption and photocatalytic degradation of enrofloxacin. Journal of Environmental Sciences. 2021; 103: 93-107. doi: 10.1016/j.jes.2020.10.006
- Hu X, Sun Y. Oxidative degradation of polycyclic aromatic hydrocarbons in industrial site contaminated soil by chlorine dioxide (Chinese). Journal of applied technology. 2021; 21(1): 88-91.
- Oleszczuk P, Kołtowski M. Effect of co-application of nano-zero valent iron and biochar on the total and freely dissolved polycyclic aromatic hydrocarbons removal and toxicity of contaminated soils. Chemosphere. 2017; 168: 1467-1476. doi: 10.1016/j.chemosphere.2016.11.100
- Dong CD, Chen CW, Kao CM, et al. Wood-Biochar-Supported Magnetite Nanoparticles for Remediation of PAH-Contaminated Estuary Sediment. Catalysts. 2018; 8(2): 73. doi: 10.3390/catal8020073
- Chen J, Dong H, Tian R, et al. Remediation of Trichloroethylene-Contaminated Groundwater by Sulfide-Modified Nanoscale Zero-Valent Iron Supported on Biochar: Investigation of Critical Factors. Water, Air, & Soil Pollution. 2020; 231(8). doi: 10.1007/s11270-020-04797-3
- Dong H, Zhang C, Hou K, et al. Removal of trichloroethylene by biochar supported nanoscale zero-valent iron in aqueous solution. Separation and Purification Technology. 2017; 188: 188-196. doi: 10.1016/j.seppur.2017.07.033
- Ouyang D, Yan J, Qian L, et al. Degradation of 1,4-dioxane by biochar supported nano magnetite particles activating persulfate. Chemosphere. 2017; 184: 609-617. doi: 10.1016/j.chemosphere.2017.05.156
- Yan J, Han L, Gao W, et al. Biochar supported nanoscale zerovalent iron composite used as persulfate activator for removing trichloroethylene. Bioresource Technology. 2015; 175: 269-274. doi: 10.1016/j.biortech.2014.10.103
- Dong CD, Chen CW, Hung CM. Synthesis of magnetic biochar from bamboo biomass to activate persulfate for the removal of polycyclic aromatic hydrocarbons in marine sediments. Bioresource Technology. 2017; 245: 188-195. doi: 10.1016/j.biortech.2017.08.204
- Li F, Lin M. Synthesis of Biochar-Supported K-doped g-C3N4 Photocatalyst for Enhancing the Polycyclic Aromatic Hydrocarbon Degradation Activity. International Journal of Environmental Research and Public Health. 2020; 17(6): 2065. doi: 10.3390/ijerph17062065
- Meng F, Song M, Chen Y, et al. Promoting adsorption of organic pollutants via tailoring surface physicochemical properties of biomass-derived carbon-attapulgite. Environmental Science and Pollution Research. 2020; 28(9): 11106-11118. doi: 10.1007/s11356-020-10974-y
Supporting Agencies
Copyright (c) 2023 Fayun Li, Meixia Lin, Xiaotong Li, Wei Wang, Kokyo Oh
License URL: https://creativecommons.org/licenses/by/4.0/

This site is licensed under a Creative Commons Attribution 4.0 International License (CC BY 4.0).
.jpg)
Beijing University of Technology, China



