
Asia Pacific Academy of Science Pte. Ltd. (APACSCI) specializes in international journal publishing. APACSCI adopts the open access publishing model and provides an important communication bridge for academic groups whose interest fields include engineering, technology, medicine, computer, mathematics, agriculture and forestry, and environment.
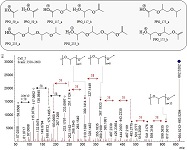
The (partial) replacement of synthetic polymers with bioplastics is due to increased production of conventional packaging plastics causing for severe environmental pollution with plastics waste. The bioplastics, however, represent complex mixtures of known and unknown (bio)polymers, fillers, plasticizers, stabilizers, flame retardant, pigments, antioxidants, hydrophobic polymers such as poly(lactic acid), polyethylene, polyesters, glycol, or poly(butylene succinate), and little is known of their chemical safety for both the environment and the human health. Polymerization reactions of bioplastics can produce no intentionally added chemicals to the bulk material, which could be toxic, as well. When polymers are used to food packing, then the latter chemicals could also migrate from the polymer to food. This fact compromises the safety for consumers, as well. The scarce data on chemical safety of bioplastics makes a gap in knowledge of their toxicity to humans and environment. Thus, development of exact analytical protocols for determining chemicals of bioplastics in environmental and food samples as well as packing polymers can only provide warrant for reliable conclusive evidence of their safety for both the human health and the environment. The task is compulsory according to legislation Directives valid to environmental protection, food control, and assessment of the risk to human health. The quantitative and structural determination of analytes is primary research task of analysis of polymers. The methods of mass spectrometry are fruitfully used for these purposes. Methodological development of exact analytical mass spectrometric tools for reliable structural analysis of bioplastics only guarantees their safety, efficacy, and quality to both humans and environment. This study, first, highlights innovative stochastic dynamics equations processing exactly mass spectrometric measurands and, thus, producing exact analyte quantification and 3D molecular and electronic structural analyses. There are determined synthetic polymers such as poly(ethylenglycol), poly(propylene glycol), and polyisoprene as well as biopolymers in bags for foodstuffs made from renewable cellulose and starch, and containing, in total within the 20,416–17,495 chemicals per sample of the composite biopolymers. Advantages of complementary employment in mass spectrometric methods and Fourier transform infrared spectroscopy is highlighted. The study utilizes ultra-high resolution electrospray ionization mass spectrometric and Fourier transform infrared spectroscopic data on biodegradable plastics bags for foodstuffs; high accuracy quantum chemical static methods, molecular dynamics; and chemometrics. There is achieved method performance |r| = 0.99981 determining poly(propylene glycol) in bag for foodstuff containing 20,416 species and using stochastic dynamics mass spectrometric formulas. The results highlight their great capability and applicability to the analytical science as well as relevance to both the fundamental research and to the industry.
Recovering of soil contaminated by hydrocarbons mixing
Vol 4, Issue 2, 2023
Download PDF
Abstract
In Mexico, an agricultural soil poor in nitrogen (N) contaminated by a hydrocarbon derivative such as automotive residual oil (ARA), with a relatively high concentration of 100,000 ppm, is an environmental problem, but also because it drastically affects soil properties associated with the mineralization of organic matter and loss of fertility, since it exceeds the maximum accepted limit of 4400 ppm of the Mexican standard called, NOM-138-SEMARNAT-2012 (NOM-138). An alternative solution is to treat it with ecological actions to eliminate the ARA and recover fertility. Therefore, the objectives of this research were: i) bioremediation of soil contaminated by 100,000 ppm of ARA ii) phytoremediation using Sorghum vulgare with Aspergillus inger and Penicillium chrysogenum to decrease ARA to a value below 4400 ppm of NOM-138. For this purpose, soil recovery was performed using the variable-response: disappearance of ARA by Soxhlet at the beginning and after bioremediation and at the end of phytoremediation with S. vulgare with phenology and biomass to seedling. All experimental data were validated by ANOVA/Tukey HSD P < 0.05%. The results indicated that bioremediation and phytoremediation of soil contaminated by 100,000 ppm of ARA, decreased it to 3400 ppm, a value lower than the maximum established by NOM-138, sufficient for soil recovery in agricultural production, in 120 days, a relatively short period of time.
Keywords
References
- Wu M, Dick WA, Li W, et al. Bioaugmentation and biostimulation of hydrocarbon degradation and the microbial community in a petroleum-contaminated soil. International Biodeterioration & Biodegradation. 2016; 107: 158-164. doi: 10.1016/j.ibiod.2015.11.019
- General law of ecological balance and environmental protection. Available online: http://www.diputados.gob.mx/Leyes Bblio/pdf/1 48 240117.pdf (accessed on 2 June 2023).
- Norma Oficial Mexicana NOM-138-SEMAR NAT/SSA1-2012, Maximum permissible limits of hydrocarbons in soils and guidelines for sampling in the characterization and specifications for remediation. DOF Secretary of the Interior (Spanish). Available online: http://www.dof.gob.mx/nota de- talle.php? codigo=5313544&fécha=10/09/2013 (accessed on 2 June 2023).
- Thapa B, KC AK, Ghimire A. A Review on bioremediation Of Petroleum Hydrocarbon Contaminants in Soil. Kathmandu University Journal of Science, Engineering and Technology. 1970; 8(1): 164-170. doi: 10.3126/kuset.v8i1.6056
- Shahi A, Aydin S, Ince B, et al. Evaluation of microbial population and functional genes during the bioremediation of petroleum-contaminated soil as an effective monitoring approach. Ecotoxicology and Environmental Safety. 2016; 125: 153-160. doi: 10.1016/j.ecoenv.2015.11.029
- Rivera Ortiz P, Rivera Lárraga JE, et al. Biostimulation and bioremediation of oil-contaminated drill cuttings (Spanish). Revista Internacional de Contaminación Ambiental. 2018; 34(2): 249-262. doi: 10.20937/rica.2018.34.02.06
- Torri SI, Cabrera MN, Alberti C. Potential respiration during biostimulation of a soil contaminated with polycyclic aromatic hydrocarbons. Rev Int Contam Ambient. 2018; 34(1):127-36. doi: 10.20937/rica.2018. 34.01.11.
- Reyes Reyes MA, Puentes Cala EA, Casanova Montes EL, López Deluque F, Panqueva Alvarez JH, Castillo Villamizar GA. Immobilization of potentially crude oil-degrading bacteria in natural and synthetic organic matrices. Rev Int Contam Ambie. 2018; 34(4): 597- 609. doi: 10.20937/RICA.2018. 34.04.04
- Guevara Espinosa MaD, Cruz Miranda N, Rivera Morales C, et al. Phytoremediation of soils contaminated with Mn and Cu from Octmum basilicum. Rev Latinoam Ambient Cienc. 2018; 9(22): 6-89.
- Leitão AL, Enguita FJ. Gibberellins in Penicilli- um strains: challenges for endophyte-plant host interactions under salinity stress. Microbiol Res. 2016; 183: 8-18. doi: 10.1016/j. micres.2015.11.004
- Solyman SNED, Abdel-Monem MO, Abou-Taleb KA, et al. Production of Plant Growth Regulators by Some Fungi Isolated under Salt Stress. South Asian Journal of Research in Microbiology. Published online March 4, 2019: 1-10. doi: 10.9734/sajrm/2019/v3i130076
- Bilal L, Asaf S, Hamayun M, et al. Plant growth promoting endophytic fungi Asprgillus fumigatus TS1 and Fusarium proliferatum BRL1 produce gibberellins and regulates plant endogenous hormones. Symbiosis 2018; 76(2):117-27. doi: 10.1007/ s13199-018-0545-4
- Garcia González MM, Farías Rodríguez R, Peña Cabriales JJ, et al. Inoculation of wheat var. Pavón with Azospirillum spp. and Azoto- bacter beijerinckii. Terra Latinoam. 2005; 23(1): 65-72.
- Contreras H, Carreño C. Efficiency of petroleum hydrocarbon biodegradation by filamentous fungi isolated from contaminated soil. Rev de Investig Agroproduccion Sustentable. 2018; 1(1): 27-33. doi: 10.25127/ ucni.vlil.269
- Hernández Valencia I, Mager D. Use of Panicum maximum and Brachiaria brizantha to phytoremediate soils contaminated with a light petroleum crude oil. Bioagro. 2003; 15(3): 149-56.
- Delgadillo López AE, González Ramírez CA, Prieto García F, et al. Phytoremediation: an alternative to eliminate contamination. Trop Subtrop Agroecosyst. 2011; 14(2): 597-612.
- Ite AE, Ibok UJ. Role of plants and microbes in bioremediation of petroleum hydrocarbons contaminated soils. Int J Environ Bioremediat Biodegrad. 2019; 7(1):1-19. doi: 10. 12691/ijebb-7-1-1
- Mexican Official Norm N OM-021-SEMARNAT-2000, which establishes the specifications of fertility, salinity and soil classification, study, sampling and analysis. Available online: http://diariooficial. gob.mXnota detalle.php?codigo=717582&fecha =31/12/2002 (accessed on 2 June 2023).
- Sánchez-Yáñez J. Brief Treatise on Agricultural Microbiology, theory and practice, Universidad Michoacana de San Nicolás de Hidalgo, Centro de Investigaciones y Desarrollo del Estado de Michoacán (Spanish). Sedagro Cosustenta, SA de CV, Morelia, Michoacan; 2007. p. 118-119.
- Baltierra Trejo E, Marquez Benavides L, Sanchez-Yáñez JM. Inconsistencies and ambiguities in calculating enzyme activity: The case of lacca se. J Microbiol Methods. 2015; 119: 126-31. doi: 10.1016/j.mimet.2015.10.007
- Walpole ER, Myers RH, Myers SL. Probability and Statistics for Engineering and Science. Available online: http://librosenpdf.org/libro-pdf-probabi lidad-y-estadistica/ (accessed on 2 June 2023).
- Riojas González HH, Gortáres Moroyoqui P, Mondaca Fernández I, et al. Influence of surfactants in the remediation of hydrocarbon-contaminated soils. Bistua. 2016; 7(1): 94-115. doi: 10.18359/ rfbb.2066
- Ramos Oseguera CA, Castro Ramírez AE, León Martínez NS, et al. Vermicomposting to recover sandy loam soil fertility and peanut (Arachis hypogaea L.) yield. Terra Latinoam. 2019; 37(1): 45-55. doi: 10.28940/tl.v37i1.331
- Jiménez Hernández V, Guerra Sánchez R. Obtaining an enriched medium to make the bioavailability of weathered hydrocarbons in a coastal soil more efficient. Rev Int Contam Ambient. 2016; 32(4): 413-24. doi: 10.20937/RICA.2016.32.04.05.
- Alvaro CES, Arocena LA, Martínez MA, et al. Aerobic biodegradation of hydrocarbon fractions from oil activity in a soil of the Northern Patagonia region, Argentina. Rev Int Contam Ambie. 2017; 33(2): 247-57. doi: 10. 2093 7/RICA.2017.33.33.02.06
- Velásquez Arias JA. Soil and water contamination by hydrocarbons in Colombia (Spanish). Análisis de la fitorremediación como estrategia biotecnológica de recuperación. Revista de Investigación Agraria y Ambiental. 2017; 8(1): 151-167. doi: 10.22490/21456453.1846
- Mohsenzadeh F, Chehregani Rad A, Akbari M. Evaluation of oil removal efficiency and enzymatic activity in some fungal strains for bioremediation of petroleum-polluted soils. Iranian Journal of Environmental Health Science & Engineering. 2012; 9(1). doi: 10.1186/1735-2746-9-26
- Barrios Ziolo LF, Robayo Gómez J, Prieto Cada-vid S, et al. Bioremediation of soils contaminated with used motor oils. Revista Cintex. 2015; 20(1): 69-96.
- Effendi AJ, Kamath R, McMillen S, et al. Strategies for Enhancing Bioremediation for Hydrocarbon-Impacted Soils. In: Proceeding of the Society of Petroleum Engineers International. Asia Pacific Health, Safety, Security, Environment and Social Responsibility Conference; 2017. doi: 10.1109/ITME.2015.163
- Leitão AL. Potential of Penicillium Species in the Bioremediation Field. International Journal of Environmental Research and Public Health. 2009; 6(4): 1393-1417. doi: 10.3390/ijerph6041393
- Chaudhary S, Shankar A, Singh A, et al. Usefulness of Penicillium in enhancing plants resistance to abiotic stresses: An overview. In: Chaudhary S, Shankar A, Singh A, et al. (editors). New and Future Developments in Microbial Biotechnology and Bioengineering. Elsevier; 2018. pp. 277-84. doi: 10.1016/ B978-0-444-63501-3.00017-X
Supporting Agencies
Copyright (c) 2023 Juna Manuel Sánchez-Yáñez
License URL: https://creativecommons.org/licenses/by/4.0/

This site is licensed under a Creative Commons Attribution 4.0 International License (CC BY 4.0).
.jpg)
Beijing University of Technology, China



