
Publication Frequency
Quarterly since 2025
Journal Articles
Search
Search scope
Volume Arrangement
2023
Featured Articles
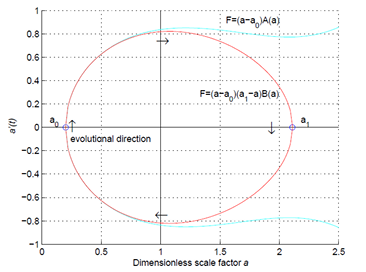
If general relativity is correct, then the origin of the universe is a simple mathematical problem. The Friedmann equation in cosmology is a well-structured ordinary differential equation, and the global properties of its solutions can be qualitatively analyzed by the phase-trajectory method. In this paper we show that the total energy density of matter in the universe is positive, and the total pressure near the Big Bang is negative. By analyzing the global properties of the solutions to the Friedmann equation according to these two conditions of state functions, we find that the Big Bang is impossible, and the space must be a closed 3-dimensional sphere, the cosmological constant is likely to be zero, and the evolution of the universe should be cyclic. The analysis and the proof are simple and straight forward, therefore these conclusions should be reliable.
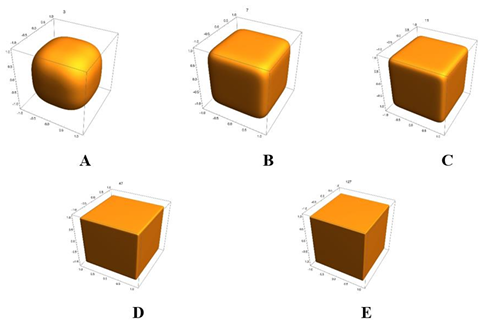
Fermat’s last theorem appears not as a unique property of natural numbers but as the bottom line of extended possible issues involving larger dimensions and powers when observed from a natural vector space viewpoint. The fabric of this general Fermat’s theorem structure consists of a well-defined set of vectors associated with dimensional vector spaces and the Minkowski norms one can define there. Here, a special vector set is studied and named a Fermat surface. Besides, a connection between Fermat surfaces and hypercubes is unveiled.
Co-dynamics of measles and hand-foot-mouth disease
Article ID: 3359
Vol 3, Issue 2, 2025
DOI: https://doi.org/10.54517/mss3359
Vol 3, Issue 2, 2025
Received: 28 February 2025; Accepted: 3 April 2025; Available online: 14 April 2025; Issue release: 30 June 2025
Download PDF
Abstract
This study develops two compartmental models to analyze the co-dynamics between measles and hand, foot, and mouth disease (HFMD): a four-compartment model and a seven-compartment HFMD-Measles co-infection model. For the four-compartment model, we systematically analyzed the co-dynamics of measles and hand, foot, and mouth disease (HFMD), and employed the next-generation matrix method to calculate the basic reproduction number of measles and that of HFMD. Through the analytical study of these two types of basic reproduction numbers, we rigorously determined the existence of the disease equilibrium points, with their quantitative relationship were clearly illustrated through graphical representations. The global asymptotic stability of these equilibria is established by applying LaSalle invariance principle, with stability regions of the four equilibrium points precisely defined. The analysis reveals that within the stability region of the disease-free equilibrium, both diseases will eventually die out, preventing any outbreaks. In the stability region corresponding to the measles equilibrium, HFMD is eliminated while measles remains endemic. Conversely, in the stability region of the HFMD-only equilibrium, measles dies out whereas HFMD persists. Finally, within the stability region of the coexistence equilibrium, both diseases persist and become endemic. Numerical simulations further validate the consistency and reliability of these theoretical results. For the seven-compartment infectious disease model, we calculated the basic reproduction number and verified the threshold theorem. We derived the conditions for both local and global asymptotic stability of the disease-free equilibrium. In particular, the disease-free equilibrium is locally stable when the basic reproduction number is less than one, and we also provided conditions for its global stability. Model validation is performed by fitting empirical data from China on HFMD and measles cases.
Keywords
co-infection; basic reproduction number; LaSalle invariance principle; global asymptotic stability
References
- Perry RT, Halsey NA. The clinical significance of measles: A review. J. Infect. Dis. 2004; 189: S4–S16.
- Vitek CR, Aduddell M, Brinton MJ, et al. Increased protections during a measles outbreak of children previously vaccinated with a second dose of measles mumps and rubella vaccine. Pediatr. Infect. Dis. J. 1999; 18(7): 620–623.
- Carazo S, Billard MN, Boutin A, De Serres G. Effect of age at vaccination on the measles vaccine effectiveness and immunogenicity: Systematic review and meta-analysis. BMC Infectious Diseases. 2020; 20: 1–18.
- Do LAH, Toh ZQ, Licciardi PV, et al. Can early measles vaccination control both measles and respiratory syncytial virus infections? The Lancet Global Health. 2022; 10(2): e288–e292.
- Azam JM, Saitta B, Bonner K, et al. Modelling the relative benefits of using the measles vaccine outside cold chain for outbreak response. Vaccine. 2021; 39(39): 5845–5853.
- Xue Y, Ruan X, Xiao Y. Measles dynamics on network models with optimal control strategies. Advances in Difference Equations. 2021; 2021(1): 138.
- Ibrahim MA, Dénes A. Stability and threshold dynamics in a seasonal mathematical model for measles outbreaks with double-dose vaccination. Mathematics. 2023; 11(8): 1791.
- Peter OJ, Panigoro HS, Ibrahim MA, et al. Analysis and dynamics of measles with control strategies: a mathematical modeling approach. International Journal of Dynamics and Control. 2023; 11(11): 2538–2552.
- Ahmad W, Butt AIK, Rafiq M, et al. Modeling, analyzing and simulating the measles transmission dynamics through efficient computational optimal control technique. European Physical Journal Plus. 2024; 139(566).
- Berhe HW, Gebremeskel AA, Atsbaha HA, et al. Modelling and stability analysis of the dynamics of measles with application to Ethiopian data. Heliyon. 2024; 10(e33594).
- Arsal SR, Aldila D, Handari BD. Short review of mathematical model of measles. AIP Conf. Proc. 2020; 2264(1): 020003.
- Ma YJ, Liu MX, Hou Q, et al. Modelling seasonal HFMD with the recessive infection in Shandong, China. Mathematical Biosciences and Engineering. 2013; 10(4): 1159–1171.
- Wang JY, Xiao YN, Peng ZH. Modelling seasonal HFMD infections with the effects of contaminated environments in Chinese mainland. Applied Mathematics and Computation. 2016; 274: 615–627.
- Wang JY, Xiao YN, Cheke RA. Modelling the effects of contaminated environments on seasonal HFMD infections and the potential benefit of a pulse vaccination strategy. Discrete & Continuous Dynamical Systems-B. 2017; 22(11): 1–22.
- Zhang TR, Zhang ZJ, Yu ZY, et al. Effects of behaviour change on HFMD transmission. Journal of Biological Dynamics. 2023; 17(1): 2244968.
- De Moraes-Pinto MI. Interaction between pediatric HIV infection and measles. Future Virology. 2011; 6(12): 1471–1479.
- Berhe HW, Makinde OD, Theuri DM. Co-dynamics of measles and dysentery diarrhea diseases with optimal control and cost-effectiveness analysis. Applied Mathematics and Computation. 2019; 347: 903–921
- Zhang J. Nursing Care for a Child with Hand-Foot-Mouth Disease Complicated by Measles and Pneumonia (In Chinese). Contemporary Nurse (Lower Fortnightly Edition). 2019; 26(04): 151–152.
- Chen S, Ran Y, Huang H, et al. Epidemic Dynamics of Two-Pathogen Spreading for Pairwise Models. Mathematics. 2022; 10: 1906.
- Smith HL, Waltman P. The theory of the chemostat: dynamics of microbial competition. Cambridge University Press; 1995.
- Lu GC, Lu ZY. Geometric criteria for the stability of high-dimensional systems (In Chinese). Science Press; 2019.
- Van den Driessche P, Watmough J. Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease transmission. Mathematical Biosciences. 2002; 180(1–2): 29–48.
- Martcheva M. An introduction to mathematical epidemiology. Springer; 2015.
- LaSalle JP. The stability of dynamical systems. SIAM; 1976.
- Thieme HR. Convergence results and a Poincaré-Bendixson trichotomy for asymptotically autonomous differential equations. J. Math. Biol. 1992; 30: 755–763.
- Zhang J, Ma Z. Global dynamics of an SEIR epidemic model with saturating contact rate. Math Biosci. 2003; (185): 15–32.
- Fandio R, Abboubakar H, Fouda HPE, et al. Mathematical modelling and projection of Buruli ulcer transmission dynamics using classical and fractional derivatives: A case study of Cameroon. Partial Differential Equations in Applied Mathematics. 2023; 8: 100589
Supporting Agencies
Copyright (c) 2025 Author(s)
License URL: https://creativecommons.org/licenses/by/4.0/
Editor-in-Chief

Prof. Youssri Hassan Youssri
Cairo University, Egypt
Indexing & Archiving
News & Announcements
2025-05-08
Prof. Rami Ahmad El-Nabulsi is highly deserving of a Lifetime Achievement Award
Prof. Rami Ahmad El-Nabulsi, Researcher at Czech Academy of Science, Czech Republic, received the Lifetime Achievement Award!
2025-03-01
Publication Frequency of MSS changes to be quarterly!
We are pleased to announce that, effective from 2025, the publication frequency of this journal will be adjusted to a quarterly schedule, with four issues released annually in March, June, September, and December....
2024-09-20
Highly Read Article Recommendation
Since the journal launched in 2023, we have been proud to publish a plethora of insightful findings in the realms of mathematics and systems science....
2024-07-10
Meeting our Editor-in-Chief and Associate Editor
It is with great pride that we introduce Prof. Youssri Hassan Youssri and Prof. Ali Akgül, who are the Editor-in-Chief and Associate Editor of our esteemed journal.
Member Application
Journal Center
Asia Pacific Academy of Science Pte. Ltd. (APACSCI) specializes in international journal publishing. APACSCI adopts the open access publishing model and provides an important communication bridge for academic groups whose interest fields include engineering, technology, medicine, computer, mathematics, agriculture and forestry, and environment.


.jpg)

.jpg)
