
Asia Pacific Academy of Science Pte. Ltd. (APACSCI) specializes in international journal publishing. APACSCI adopts the open access publishing model and provides an important communication bridge for academic groups whose interest fields include engineering, technology, medicine, computer, mathematics, agriculture and forestry, and environment.
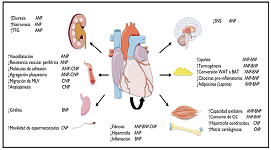
Myocardial support
Vol 4, Issue 2, 2023
Download PDF
Abstract
Objective: The cardiac muscle cannot be anatomically free in the thorax and without a support to fulfill its hemodynamic function. Therefore, the possibility of the existence of a support point acting as a lever was analyzed. Material and methods: We used: 1) cardiac dissection in ten young bovine hearts [800-1000 g]; 2) cardiac dissection in eight human hearts: one embryo of 23 weeks gestation; one 10 year old, 250 g; and six adults, mean weight 300 g. The myocardial band was unwound completely. The myocardial band was uncoiled in its entirety. The extracted pieces were analyzed for anatomy and histology. The investigation was completed with simple radiographic imaging studies, magnetic resonance imaging and computed tomography. Results: In anatomical investigations we have found in all human and bovine hearts studied a nucleus underlying the right trigone of bone-chondroid-tendinous histological structure. Microscopic analysis revealed in bovine hearts a trabecular osteochondral matrix [fulcrum]. In all human hearts the fulcrum was found to be formed by chondroid tissue. In this structure, not described by other authors, the origin and end of the myocardial fibers have muscular insertion. Imaging techniques confirmed its existence. Conclusions: The cardiac fulcrum found in the anatomical investigation of human and bovine hearts would clarify about the necessary fulcrum of the myocardial muscle to complete its twisting movements.
Keywords
References
- Henson RE, Song SK, Pastorek JS, et al. Left ventricular torsion is equal in mice and humans. Am J Physiol Heart Circ Physiol. 2000; 278: H1117-H1123.
- Trainini J, Lowenstein J, Beraudo M, et al. Myocardial Torsion. Ed Biblos Buenos Aires; Argentina; 2019.
- Pettigrew JB. On the arrangement of the muscular fibres in the ventricles of the vertebrate heart with phisiological remarks. Philos Trans. 1864; 154: 445-500.
- Shaner RF. On the muscular architecture of the vertebrate ventricle. J Anat. 1923; 58: 59-70.
- Torrent-Guasp F, Kocica MJ, Corno AF, et al. Towards new understanding of the heart structure and function. Eur J Cardiothorac Surg. 2005; 27: 191-201.
- Torrent Guasp F Buckberg G, Carmine C, et al. The structure and function of the helical heart and its buttress wrapping. I. The normal macroscopic structure of the heart. Semin Thorac Cardiovasc Surg. 2001; 13: 301-319.
- Maclver DH, Stephenson RS, Jensen B, et al. The end of the unique myocardial band: Part I. Anatomical considerations. Eur J Cardiothorac Surg. 2018; 53: 112-119. doi: 10.1093/ejcts/ezx290
- Maclver DH, John B. Partridge JB, et al. The end of the unique myocardial band: Part II. Clinical and functional considerations. Eur J Cardio-Thoracic Surg. 2018; 53: 120-128.
- Elencwajg B, Lopez-Cabanillas N, Cardinali EL, et al. The Jurdham procedure endocardial left ventricular lead insertion via a femoral transseptal sheath for cardiac resynchronization therapy pectoral device implantation. Heart Rhythm. 2012; 9: 1798-1804.
- Trainini JC, Herreros J, Elencwajg B, et al. Myocardial dissection. Rev Argent Cardiol. 2017; 85: 44-50.
- Moittié S, Baiker K, Strong V, et al. Discovery of os cordis in the cardiac skeleton of chimpanzees [Pan troglodytes]. Scientific Reports. 2020; 10: 9417.
- Anderson R, Ho S, Redman K, et al. The anatomical arrangement of the myocardial cells making up the ventricular mass. Eur J Cardiothoracic Surg. 2005; 28: 517-525.
- Trainini JC, Trainini A, Valle Cabezas J, et al. Left Ventricu lar Suction in Right Ventricular Dysfunction. EC Cardiology. 2019; 6: 572-577.
- Mora V, Roldán I. Romero E, et al. Myocardial contraction during the diastolic isovolumetric period: analysis of longitudinal strain by means of speckle tracking echocardiography. J Cardiovasc Dev Dis. 2018; 5: 4.
- Trainini JC, Elencwajg B, Herreros J. New Physiological Concept of the Heart. Ann Transplant Res. 2017; 1: 1001.
- Carreras F, Ballester M, Pujadas S, et al. Morphological and functional evidences of the helical heart from non-invasive cardiac imaging. Eur J Cardiothoracic Surg. 2006; 29[Suppl 1]: S50-S55.
- Ballester M, Ferreira A, Carreras F. The myocardial band. Heart Fail Clin. 2008; 4: 261-272.
- Kocica MJ, Corno AF, Carreras-Costa F, et al. The helical ventricular myocardial band: global, three-dimensional, functional architecture of the ventricular myocardium. Eur J Cardiothorac Surg. 2006; 29(Suppl-I): S21-S40.
Supporting Agencies
Copyright (c) 2023 Jorge Trainini, Mario Beraudo, Mario Wernicke, Alejandro Trainini, Diego Haber Lowenstein, María Elena Bastarrica, Darío Carlos Martino, Jorge Lowenstein

This work is licensed under a Creative Commons Attribution 4.0 International License.

This site is licensed under a Creative Commons Attribution 4.0 International License (CC BY 4.0).

Prof. Prakash Deedwania
University of California,
San Francisco, United States




