
Asia Pacific Academy of Science Pte. Ltd. (APACSCI) specializes in international journal publishing. APACSCI adopts the open access publishing model and provides an important communication bridge for academic groups whose interest fields include engineering, technology, medicine, computer, mathematics, agriculture and forestry, and environment.
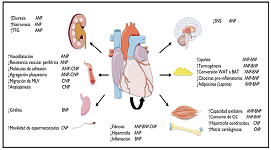
Heart as an endocrine-metabolic organ
Vol 4, Issue 2, 2023
Download PDF
Abstract
Historically, the heart has been considered a vital organ due to its function as an impeller and aspirating blood pump, allowing the fi siological perfusion to every corner of the human body. However, behind this pump role lies a little-discussed function, that is, the endocrine-metabolic role of the heart, as a hormone synthesizing organ and, because of its pump function, a distributor of hormones foreign to it. Understanding the incidence of peptides synthesized in the heart offers a new perspective on the relevance of the heart in homeostasis and how cardiovascular disease can alter metabolism. The present work aims to review the most current information on the role of natriuretic peptides produced in the heart and their endocrine-metabolic function. These are atrial, brain and C-type natriuretic peptides. It is concluded that the conception of the heart as an organ of endocrine-metabolic regulation, whose hormones are essential for the homeostasis of the organism, is fundamental for the medicine of this century and for future advances in new therapeutic strategies to improve the quality of life of patients.
Keywords
References
- Cruz Ortega HA, Calderón Monter FX. The heart and its normal and aggregate heart sounds. A brief review of the subject. Rev Fac Med UNAM. 2016; 59: 49–55.
- Saturno Chiu G. Cardiology, 1st ed. Bogotá, DC: Editorial El Manual Moderno; 2017.
- Silva Furtado DF, Prata Vasconcelos LD, Branco É, de Lima AR. Cardiac anatomy and aortic branching in the capuchin monkey (Sapajus apella) (Portuguese). Biotemas. 2017; 30: 83–93.
- Vera M, Huérfano Y, Valbuena O, et al. Automatic segmentation of the left atrium in cardiac computed tomography images. Rev Latinoam Hipertens. 2016; 11: 54–59.
- Lowe JS, Anderson PG, Anderson SI. Human Histology, 5th ed. Spain: Elsevier; 2020.
- Mescher AL. Junqueira’s Basic Histology Text and Atlas, 15th ed. New York: LANGE; 2018.
- Mohrman DE, Heller LJ. Cardiovascular Physiology, 9th ed. New York: LANGE; 2018. p. 368.
- Castillo Moya A, del Pozo Bascuñán P. Cardiopulmonary interactions: From physiology to clinic. Rev Chil Pediatr. 2018; 89: 582–591.
- Pérez González LL, González Escudero M, Pérez Acosta ND. Peptides and their usefulness in clinical practice. Panorama Cuba y Salud. 2018; 13: 78–82.
- Rademaker MT, Scott NJA, Koh CY, et al. Natriuretic peptide analogues with distinct vasodilatory or renal activity: integrated effects in health and experimental heart failure. Cardiovasc Res. 2021; 117: 508–519.
- Idzikowska K, Zielinska M. Midregional proatrial natriuretic peptide, an important member of the natriuretic peptide family: potential role in diagnosis and prognosis of cardiovascular disease. J Int Med Res. 2018; 46: 3017–3029.
- Cannone V, Cabassi A, Volpi R, et al. Atrial natriuretic peptide: A molecular target of novel therapeutic approaches to cardio-metabolic disease. Int J Mol Sci. 2019; 20: 3265–3274.
- Tanase DM, Radu S, Al Shurbaji S, et al. Natriuretic peptides in heart failure with preserved left ventricular ejection fraction: From molecular evidences to clinical implications. Int J Mol Sci. 2019; 20: 1–23.
- Nakano SJ, Everitt MD. Neurohormonal axis and natriuretic peptides in heart failure. In: Heart failure in the child and young adult: from bench to bedside, 1st ed. Elsevier Inc; 2018. pp. 75–86.
- Salas GL, Jozefkowicz M, Goldsmit GS, et al. B-type natriuretic peptide: utility in the management of critically ill newborns. Arch Argent Pediatr. 2017; 115: 483–489.
- González Sánchez JA, Sánchez E, Contreras M. Natriuretic peptide (BNP). Rev Soc Venez Med Interna. 2018; 34: 184–188.
- Pascual-Figal DA, Casademont J, Lobos JM, et al. Consensus document and recommendations on the use of natriuretic peptides in clinical practice. Rev Clin Esp. 2016; 216: 313–322.
- Lorente DM, Hernández M, Arocena MJ, et al. Use of NT-pro BNP as a predictor of evolution in postoperative cardiac surgery. Rev Uruguaya Cardiol. 2019; 34: 240–247.
- Vasquez N, Carter S, Grodin JL. Angiotensin receptor -neprilysin inhibitors and the natriuretic peptide axis. Curr Heart Fail Rep. 2020; 17: 67–76.
- Escobar-Guerrero D, Suárez-Jaramillo A, Ullauri-Solórzano V, et al. Sensitivity of NT-pro- BNP compared with echocardiogram ejection fraction in patients with suspected systolic left heart failure. Rev Ecuatoriana Cardiol. 2016; 2: 1–10.
- Moyes AJ, Hobbs AJ. C-Type natriuretic peptide: a multifaceted paracrine regulator in the heart and vasculature. Int J Mol Sci. 2019; 20: 2281–2295.
- Spiranec K, Chen W, Werner F, et al. Endothelial C-type natriuretic peptide acts on pericytes to regulate microcirculatory flow and blood pressure. Circulation. 2018; 138: 494–508.
- Wilson MO, Barrell GK, Prickett TCR, et al. Molecular forms of C-type natriuretic peptide in cerebrospinal fluid and plasma reflect differential processing in brain and pituitary tissues. Peptides. 2018; 99: 223–230.
- Navarro Solano J, Poveda Fernández J. Update on the use of neprilysin and angiotensin II receptor inhibitors: ripple in the benefit on different entities. Rev Costarric Cardiol. 2018; 20: 22–36.
- Jerez Castro AM. Biomarkers in heart failure. Soc Cuba Cardiol. 2019; 11: 317–325.
- Lugnier C, Meyer A, Charloux A, et al. The endocrine function of the heart: physiology and involvements of natriuretic peptides and cyclic nucleotide phosphodiesterases in heart failure. J Clin Med. 2019; 8: 1–20.
- Rignault-Clerc S, Bielmann C, Liaudet L, et al. Natriuretic Peptide Receptor B modulates the proliferation of the cardiac cells expressing the Stem Cell Antigen-1. Sci Rep. 2017; 7: 1–14.
- Craigie E, Mullins JJ, Bailey MA. Glucocorticoids and mineralocorticoids. Cardiovascular Hormone Systems: From Molecular Mechanisms to Novel Therapeutics. 2009; 25: 1–37.
- Rubattu S, Sciarretta S, Morriello A, et al. NPR-C: A component of the natriuretic peptide family with implications in human diseases. J Mol Med. 2010; 88: 889–897.
- Madiraju P, Hossain E, Anand-Srivastava MB. Natriuretic peptide receptor-C activation attenuates angiotensin II-induced enhanced oxidative stress and hyperproliferation of aortic vascular smooth muscle cells. Mol Cell Biochem. 2018; 448: 77–89.
- Tsutamoto T, Sakai H, Yamamoto T, et al. Heart is the target organ of endogenous cardiac natriuretic peptides. Int Heart J. 2020; 61: 77–82.
- Matsuo A, Nagai-Okatani C, Nishigori M, et al. Natriuretic peptides in human heart: novel insight into their molecular forms, functions, and diagnostic use. Peptides. 2019; 3–17.
- Ramos H. Acute myocardial infarction: When peptides come marching in. Rev Fed Argentina Cardiol. 2016; 45: 108–109.
- Domondon M, Nikiforova AB, DeLeon-Pennell KY, Ilatovskaya DV. Regulation of mitochondria function by natriuretic peptides. Am J Physiol Renal Physiol. 2019; 317: F1164–F1168.
- Vasile VC, Jaffe AS. Natriuretic peptides and analytical barriers. Clin Chem. 2017; 63: 50–58.
- Forte M, Madonna M, Schiavon S, et al. Cardiovascular pleiotropic effects of natriuretic peptides. Int J Mol Sci. 2019; 20.
- Lee HS, Cho KW, Kim HY, et al. Chamber-specific regulation of atrial natriuretic peptide secretion in cardiac hypertrophy: atrial wall dynamics in the ANP secretion. Pflugers Arch Eur J Physiol. 2020. doi: 10.1007/s00424-020-02377-2
- Sun JZ, Chen SJ, Majid-Hasan E, et al. Dietary salt supplementation selectively downregulates NPR-C receptor expression in kidney independently of ANP. Am J Physiol Renal Physiol. 2002; 282: 220–227.
- Sun JZ, Chen SJ, Li G, et al. Hypoxia reduces atrial natriuretic peptide peptide clearance receptor gene expression in ANP knockout mice. Am J Physiol Lung Cell Mol Physiol. 2000; 279: 511–519.
- Feng JA, Perry G, Mori T, et al. Pressure-independent enhancement of cardiac hypertrophy in atrial natriuretic peptide-deficient mice. Clin Exp Pharmacol Physiol. 2003; 30: 343–349.
- Santhekadur PK, Kumar DR Seneshaw M, et al. The multifaceted role of natriuretic peptides in metabolic syndrome. Biomed Pharmacother. 2017; 92: 826–835.
- Kinoshita H, Kuwahara K, Nishida M, et al. Inhibition of TRPC6 channel activity contributes to the antihypertrophic effects of natriuretic peptides- guanylyl cyclase-a signaling in the heart. Circ Res. 2010; 106: 1849–1860.
- He X, Li S, Liu B, et al. Major contribution of the 3/6/7 class of TRPC channels to myocardial ischemia/reperfusion and cellular hypoxia/reoxygenation injuries. Proc Natl Acad Sci U S A. 2017; 114: E4582–E4591.
- Kapoun AM, Liang F, Young G, et al. B-Type natriuretic peptide exerts broad functional opposition to transforming growth factor-β in primary human cardiac fibroblasts: fibrosis, myofibroblast conversion, proliferation, and inflammation. Circ Res. 2004; 94: 453–461.
- Fu S, Ping P, Wang F, et al. Synthesis, secretion, function, metabolism and application of natriuretic peptides in heart failure. J Biol Eng. 2018; 12: 1–21.
- Cresci S, Pereira NL, Ahmad F, et al. Heart failure in the era of precision medicine: a scientific statement from the American Heart Association. Circ Genomic Precis Med. 2019; 12: 458–485.
- Michel L, Mincu RI, Mahabadi AA, et al. Troponins and brain natriuretic peptides for the prediction of cardiotoxicity in cancer patients: a meta-analysis. Eur J Heart Fail. 2020; 22: 350–361.
- Nakagawa Y, Nishikimi T, Kuwahara K. Atrial and brain natriuretic peptides: hormones secreted from the heart. Peptides. 2019; 111: 18–25.
- Gupta DK, Wang TJ. Natriuretic peptides and cardiometabolic health. Circ J. 2015; 79: 1648–1655.
- Yat Wong PC, Guo J, Zhang A. The renal and cardiovascular effects of natriuretic peptides. Adv Physiol Educ. 2017; 41: 179–185.
- Sciarretta S, Marchitti S, Bianchi F, et al. C2238 atrial natriuretic peptide molecular variant is associated with endothelial damage and dysfunction through natriuretic peptide receptor C signaling. Circ Res. 2013; 112: 1355–1364.
- Scarpino S, Marchitti S, Stanzione R, et al. Reactive oxygen species-mediated effects on vascular remodeling induced by human atrial natriuretic peptide T2238C molecular variant in endothelial cells in vitro. J Hypertens. 2009; 27: 1804–1813.
- Rubattu S, Sciarretta S, Marchitti S, et al. The T2238C human atrial natriuretic peptide molecular variant and the risk of cardiovascular diseases. Int J Mol Sci. 2018; 19: 1–10.
- Strisciuglio T, Barbato E, De Biase C, et al. T2238C Atrial Natriuretic Peptide Gene Variant and the Response to Antiplatelet Therapy in Stable Ischemic Heart Disease Patients. J Cardiovasc Transl Res. 2018; 11: 36–41.
- Weber NC, Blumenthal SB, Hartung T, et al. ANP inhibits TNF-alpha-induced endothelial MCP-1 expression—Involvement of p38 MAPK and MKP-1. J Leukoc Biol. 2003; 74: 932–941.
- Scotland RS, Cohen M, Foster P, et al. C-type natriuretic peptide inhibits leukocyte recruitment and platelet-leukocyte interactions via suppression of P-selectin expression. Proc Natl Acad Sci U S A. 2005; 102: 14452–14457.
- Ikeda M, Kohno M, Yasunari K, et al. Natriuretic peptide family as a novel antimigration factor of vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 1997; 17: 731–736.
- Burtenshaw D, Cahill PA. Natriuretic Peptides and the Regulation of Retinal Neovascularization. Arterioscler Thromb Vasc Biol. 2020; 40: 7–10.
- Sangaralingham SJ, McKie PM, Ichiki T, et al. Circulating C-type natriuretic peptide and its relationship to cardiovascular disease in the general population. Hypertension. 2015; 65: 1187–1194.
- De Arriba de la Fuente G, Pérez del Valle KM, Gaitan Tocora DG, Rodríguez Puyol D. Arterial hypertension and kidney. Medicine. 2019; 12: 4759–4764.
- Volpe M, Carnovali M, Mastromarino V. The natriuretic peptides system in the pathophysiology of heart failure: From molecular basis to treatment. Clin Sci. 2016; 130: 57–77.
- Theilig F, Wu Q. ANP-induced signaling cascade and its implications in renal pathophysiology. Am J Physiol - Ren Physiol. 2015; 308: F1047–F1055.
- Ogawa T, de Bold AJ. The heart as an endocrine organ. Endocr Connect. 2014; 3: 1–14.
- Lima M, Carmelo Nuccio J, Villalobos M, et al. Renin angiotensin system and cardio-metabolic risk. Rev Venez Endocrinol Metab. 2010; 8: 3–10.
- Nakagawa H, Oberwinkler H, Nikolaev VO, et al. Atrial natriuretic peptide locally counteracts the deleterious effects of cardiomyocyte mineralocorticoid receptor activation. Circ Hear Fail. 2014; 7: 814–821.
- Matsukawa T, Miyamoto T. Angiotensin II-stimulated secretion of arginine vasopressin is inhibited by atrial natriuretic peptide in humans. Am J Physiol Regul Integr Comp Physiol. 2011; 300: 624–629.
- Cha SA, Park BM, Gao S, et al. Stimulation ofANP by angiotensin-(1-9) via the angiotensin type 2 receptor. Life Sci. 2013; 93: 934–940.
- Saito K, Uchino S, Fujii T, et al. Effect of low-dose atrial natriuretic peptide in critically ill patients with acute kidney injury: a retrospective, single-center study with propensity-score matching. BMC Nephrol. 2020; 21: 31–42.
- Brenner BM, Ballermann BJ, Gunning ME, Zeidel ML. Diverse biological actions of atrial natriuretic peptide. Physiol Rev. 1990; 70: 665–699.
- Armaly Z, Assady S, Abassi Z. Corin: A new player in the regulation of salt-water balance and blood pressure. Curr Opin Nephrol Hypertens. 2013; 22: 713–722.
- Zhou Y, Wu Q. Corin in natriuretic peptide processing and hypertension topical collection on mediators, mechanisms, and pathways in tissue injury. Curr Hypertens Rep. 2014; 16: 415–421.
- Tarjan E, Denton DA, Weisinger RS. Atrial natriuretic peptide inhibits water and sodium intake in rabbits. Regul Pept. 1988; 23: 63–75.
- Bell C. Regulation of metabolism. In: Primer on the Autonomic Nervous System. Elsevier; 2012. p. 253–255.
- Romero Aguilar L, Guerra Sánchez G, Pardo JP, Luqueño Bocardo OI. Lipid bodies: metabolically active organelles. Rev Educ Biochemistry. 2017; 35: 115–124.
- Chabowski A, Górski J. Muscle lipid metabolism. Muscle Exerc Physiol. 2018; 271–284.
- García Díaz JD, Mesa Latorre JM, Valbuena Parra AR, Corps Fernández D. Disorders of lipid metabolism. Medicine. 2016; 12: 1059–1071.
- Wang TJ. The natriuretic peptides and fat metabolism. N Engl J Med. 2012; 367: 377–378.
- Bartels ED, Nielsen JM, Bisgaard LS, et al. Decreased expression of natriuretic peptides associated with lipid accumulation in cardiac ventricle of obese mice. Endocrinology. 2010; 151: 5218–5225.
- Chiba A, Watanabe-Takano H, Miyazaki T, Mochizuki N. Cardiomyokines from the heart. Cell Mol Life Sci. 2018; 75: 1349–1362.
- Sengenès C, Bouloumié A, Hauner H, et al. Involvement of a cGMP- dependent pathway in the natriuretic peptide-mediated hormone-sensitive lipase phosphorylation in human adipocytes. J Biol Chem. 2003; 278: 48617–48626.
- Sengenés C, Berlan M, De Glisezinski I, et al. Natriuretic peptides: a new lipolytic pathway in human adipocytes. FASEB J. 2000; 14: 1345–1351.
- Birkenfeld AL, Boschmann M, Moro C, et al. Β-Adrenergic and Atrial Natriuretic Peptide Interactions on Human Cardiovascular and Metabolic Regulation. J Clin Endocrinol Metab. 2006; 91: 5069–5075.
- Moro C, Pillard F, De Glisezinski I, et al. Training enhances ANP lipid-mobilizing action in adipose tissue of overweight men. Med Sci Sports Exerc. 2005; 37: 1126–1132.
- Thomsen CF, Ried-Larsen M, Goetze JP, et al. Plasma proatrial natriuretic peptide associates with lipid oxidation during exercise and cardiorespiratory fitness in healthy young adults. Peptides. 2019; 122: 170156–170163.
- Cedikova M, Kripnerová M, Dvorakova J, et al. Mitochondria in White, Brown, and Beige Adipocytes. Stem Cells Int. 2016; 2016.
- González N, Moreno-Villegas Z, González-Bris A, et al. Regulation of visceral and epicardial adipose tissue for preventing cardiovascular injuries associated with obesity and diabetes. Cardiovasc Diabetol. 2017; 16: 1–11.
- Morales González F, Jimenez Badilla J. Adipose tissue as an endocrine organ: morbidity model in metabolic syndrome among others. Rev Clín Esc Med UCR-HSJD. 2018; 1–6.
- Peirce V, Carobbio S, Vidal-Puig A. The different shades of fat. Nature. 2014; 510: 76–83.
- Garcia Rosa ML, Chung Kang H, Lagoeiro Jorge AJ, et al. Role of adipose tissue in obesity and heart failure. Insufic Card. 2019; 14: 55–63.
- Muoio DM. Metabolism and Vascular Fatty Acid Transport. N Engl J Med. 2010; 363: 291–293.
- Bordicchia M, Liu D, Amri EZ, et al. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J Clin Invest. 2012; 122: 1022–1036.
- Bordicchia M, Spannella F, Ferretti G, et al. PCSK9 is expressed in human visceral adipose tissue and regulated by insulin and cardiac natriuretic peptides. Int J Mol Sci. 2019; 20: 1–15.
- Shi F, Collins S. Second messenger signaling mechanisms of the brown adipocyte thermogenic program: An integrative perspective. Horm Mol Biol Clin Investig. 2017; 31: 1–9.
- Jankovic A, Golic I, Markelic M, et al. Two key temporally distinguishable molecular and cellular components of white adipose tissue browning during cold acclimation. J Physiol. 2015; 593: 3267–3280.
- Miyashita K, Itoh H, Tsujimoto H, et al. Natriuretic peptides/cGMP/cGMP-dependent protein kinase cascades promote muscle mitochondrial biogenesis and prevent obesity. Diabetes. 2009; 58: 2880–2892.
- Zois NE, Bartels ED, Hunter I, et al. Natriuretic peptides in cardiometabolic regulation and disease. Nat Rev Cardiol. 2014; 11: 403–412.
- Engeli S, Birkenfeld AL, Badin PM, et al. Natriuretic peptides enhance the oxidative capacity of human skeletal muscle. J Clin Invest. 2012; 122: 4675–4679.
- Moro C, Klimcakova E, Lolmède K, et al. Atrial natriuretic peptide inhibits the production of adipokines and cytokines linked to inflammation and insulin resistance in human subcutaneous adipose tissue. Diabetologia. 2007; 50: 1038–1047.
- Heinisch BB, Vila G, Resl M, et al. B-type natriuretic peptide (BNP) affects the initial response to intravenous glucose: A randomised placebo-controlled cross-over study in healthy men. Diabetologia. 2012; 55: 1400–1405.
- Vila G, Grimm G, Resl M, et al. B-type natriuretic peptide modulates ghrelin, hunger, and satiety in healthy men. Diabetes. 2012; 61: 2592–2596.
- Yasoda A, Ogawa Y, Suda M, et al. Natriuretic Peptide Regulation of Endochondral Ossification. J Biol Chem. 1998; 273: 11695–11700.
- Wu K, Mei C, Chen Y, et al. C-type natriuretic peptide regulates sperm capacitation by the cGMP/PKG signalling pathway via Ca 2+ influx and tyrosine phosphorylation. Reprod Biomed Online. 2019; 38: 289–299.
- Cannone V, Buglioni A, Sangaralingham SJ, et al. Aldosterone, Hypertension, and Antihypertensive Therapy: Insights from a General Population. Mayo Clin Proc. 2018; 93: 980–990.
- Buglioni A, Cannone V, Cataliotti A, et al. Circulating aldosterone and natriuretic peptides in the general community relationship to cardiorenal and metabolic disease. Hypertension. 2015; 65: 45–53.
- Rubattu S, Forte M, Marchitti S, et al. Molecular implications of natriuretic peptides in the protection from hypertension and target organ damage development. Int J Mol Sci. 2019; 20: 798.
- Mueller C, McDonald K, de Boer RA, et al. Heart Failure Association of the European Society of Cardiology practical guidance on the use of natriuretic peptide concentrations. Eur J Heart Fail. 2019; 21: 715–731.
- Mogensen UM, Gong J, Jhund PS, et al. Effect of sacubitril/valsartan on recurrent events in the Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure trial (PARADIGM-HF). Eur J Heart Fail. 2018; 20: 760–768.
Supporting Agencies
Copyright (c) 2023 Triana-Alcívar Héctor, Ureta-Pilligua Jordy, Mera-Flores Ronny Richard, Bermúdez Cevallos Leonardo, Rivadeneira Mendoza Yokasta

This work is licensed under a Creative Commons Attribution 4.0 International License.

This site is licensed under a Creative Commons Attribution 4.0 International License (CC BY 4.0).

Prof. Prakash Deedwania
University of California,
San Francisco, United States




