
Asia Pacific Academy of Science Pte. Ltd. (APACSCI) specializes in international journal publishing. APACSCI adopts the open access publishing model and provides an important communication bridge for academic groups whose interest fields include engineering, technology, medicine, computer, mathematics, agriculture and forestry, and environment.
Renal lithiasis and cardiovascular risk
Vol 4, Issue 1, 2023
Download PDF
Abstract
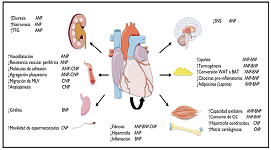
Renal lithiasis (RL) is a common disease whose prevalence has increased in recent years. It is now considered a systemic pathology, not limited to the kidney and urinary tract, but largely related to diabetes mellitus, obesity, arterial hypertension, hyperuricemia, hypercholesterolemia, and chronic kidney disease, all cardiovascular risk factors that are often linked to severe events such as stroke, coronary heart disease, or acute myocardial infarction. Numerous crosssectional studies and meta-analyses have demonstrated the association between these two entities. In this review we will attempt to demonstrate the mechanisms involved in the pathophysiology of RL and its relationship with cardiovascular disease. As mechanisms involved, three associations are mentioned. The first refers to oxidative stress and inflammation. The second association refers to the presence of lithogenic mechanisms that contribute to vascular calcification. The last theory is the already known association of obesity, metabolic syndrome, diabetes and HT, all risk factors for the development of RL as well as cardiovascular disease, remembering that RL is the cause, in 8%, of the development of chronic kidney disease, another risk factor for cardiovascular disease and death. In conclusion, the theory that RL is not a disease limited to the kidney and urinary tract, but a systemic disease, with a risk of cardiovascular events so severe that they can lead to death, is confirmed
Keywords
References
- Liu Y, Li S, Zeng Z, et al. Kidney Stones and Cardiovascular Risk: A Meta-analysis of Cohort Studies. American Journal of Kidney Diseases. 2014; 64(3): 402-410. doi: 10.1053/j.ajkd.2014.03.017
- Scales CD, Smith AC, Hanley JM, et al. Prevalence of Kidney Stones in the United States. European Urology. 2012; 62(1): 160-165. doi: 10.1016/j.eururo.2012.03.052
- Serio A, Fraioli A. Epidemiology of Nephrolithiasis. Nephron. 1998; 81(Suppl. 1): 26-30. doi: 10.1159/000046295
- Pinduli I, Spivacow R, del Valle E, et al. Prevalence of urolithiasis in the autonomous city of Buenos Aires, Argentina. Urological Research. 2006; 34(1): 8-11. doi: 10.1007/s00240-005-0003-7
- Sakhaee K. Nephrolithiasis as a systemic disorder. Current Opinion in Nephrology and Hypertension. 2008; 17(3): 304-309. doi: 10.1097/mnh.0b013e3282f8b34d
- Elmfeldt D, Vedin A, Wilhelmsson C, et al. Morbidity in representative male survivors of myocardial infarction compared to representative population samples. J Chronic Dis. 1976; 29(4): 221-231.
- Hu C, Chen Y, Huang P, et al. The association between urinary calculi and increased risk of future cardiovascular events: A nationwide population-based study. J Cardiol. 2016; 67(5): 463-470.
- Madore F, Stampfer MJ, Rimm EB, et al. Nephrolithiasis and risk of hypertension. Am J Hypertens. 1998; 11(1 Pt 1): 46-53.
- Madore F, Stampfer MJ, Willett WC, et al. Nephrolithiasis and risk of hypertension in women. Am J Kidney Dis. 1998; 32(5): 802-807.
- Fabris A, Ferraro PM, Comellato G, et al. The relationship between calcium kidney stones, arterial stiffness and bone density: unraveling the stone-bone-vessel liaison. Journal of Nephrology. 2014; 28(5): 549-555. doi: 10.1007/s40620-014-0146-0
- Kim S, Chang Y, Sung E, et al. Association Between Sonographically Diagnosed Nephrolithiasis and Subclinical Coronary Artery Calcification in Adults. American Journal of Kidney Diseases. 2018; 71(1): 35-41. doi: 10.1053/j.ajkd.2017.06.026
- Randall A. The origin and growth of renal calculi. Ann Surg. 1937; 105(6): 1009-1027.
- Weller RO, Nester B, Cooke SAR. Calcification in the human renal papilla: An electron-microscope study. The Journal of Pathology. 1972; 107(3): 211-216. doi: 10.1002/path.1711070308
- Stoller ML, Meng MV, Abrahams HM, et al. The Primary Stone Event: A New Hypothesis Involving a Vascular Etiology. Journal of Urology. 2004; 171(5): 1920-1924. doi: 10.1097/01.ju.0000120291.90839.49
- Evan AP. Physiopathology and etiology of stone formation in the kidney and the urinary tract. Pediatric Nephrology. 2009; 25(5): 831-841. doi: 10.1007/s00467-009-1116-y
- Evan AP, Lingeman JE, Coe FL, et al. Randall’s plaque of patients with nephrolithiasis begins in basement membranes of thin loops of Henle. Journal of Clinical Investigation. 2003; 111(5): 607-616. doi: 10.1172/jci17038
- Khan SR. Crystal-induced inflammation of the kidneys: results from human studies, animal models, and tissue- culture studies. Journal of Clinical and Experimental Nephrology. 2004; 8(2). doi: 10.1007/s10157-004-0292-0
- Baggio B, Gambaro G, Ossi E, et al. Increased urinary excretion of renal enzymes in idiopathic calcium oxalate nephrolithiasis. J Urol. 1983; 129(6): 1161-1162.
- Thamilselvan V, Menon M, Thamilselvan S. Oxalate-induced activation of PKC-α and -δ regulates NADPH oxidase-mediated oxidative injury in renal tubular epithelial cells. American Journal of Physiology-Renal Physiology. 2009; 297(5): F1399-F1410. doi: 10.1152/ajprenal.00051.2009
- Zuo J, Khan A, Glenton PA, et al. Effect of NADPH oxidase inhibition on the expression of kidney injury molecule and calcium oxalate crystal deposition in hydroxy-L-proline-induced hyperoxaluria in the male Sprague- Dawley rats. Nephrology Dialysis Transplantation. 2011; 26(6): 1785-1796. doi: 10.1093/ndt/gfr035
- Khan SR. Hyperoxaluria-induced oxidative stress and antioxidants for renal protection. Urological Research. 2005; 33(5): 349-357. doi: 10.1007/s00240-005-0492-4
- Toblli JE, Cao G, Casas G, et al. NF-κB and chemokine-cytokine expression in renal tubulointerstitium in experimental hyperoxaluria. Role of the renin-angiotensin system. Urological Research. 2005; 33(5): 358-367. doi: 10.1007/s00240-005-0484-4
- Umekawa T, Chegini N, Khan SR. Increased expression of monocyte chemoattractant protein-1 (MCP-1) by renal epithelial cells in culture on exposure to calcium oxalate, phosphate and uric acid crystals. Nephrology Dialysis Transplantation. 2003; 18(4): 664-669. doi: 10.1093/ndt/gfg140
- Umekawa T, Iguchi M, Uemura H, Khan SR. Oxalate ions and calcium oxalate crystal-induced up-regulation of osteopontin and monocyte chemoattractant protein-1 in renal fibroblasts. BJU Int. 2006; 98(3): 656-660.
- Khan SR. Is oxidative stress, a link between nephrolithiasis and obesity, hypertension, diabetes, chronic kidney disease, metabolic syndrome? Urological Research. 2012; 40(2): 95-112. doi: 10.1007/s00240-011-0448-9
- Rodriguez-Iturbe B, Vaziri ND, Johnson RJ. Inflammation, Angiotensin II, and Hypertension. Hypertension. 2008; 52(5). doi: 10.1161/hypertensionaha.108.121145
- Chung SSM, Ho ECM, Lam KSL, et al. Contribution of Polyol Pathway to Diabetes-Induced Oxidative Stress. Journal of the American Society of Nephrology. 2003; 14(suppl_3): S233-S236. doi: 10.1097/01.asn.0000077408.15865.06
- Manea A. NADPH oxidase-derived reactive oxygen species: involvement in vascular physiology and pathology. Cell and Tissue Research. 2010; 342(3): 325-339. doi: 10.1007/s00441-010-1060-y
- Steckelings UM, Rompe F, Kaschina E, et al. The evolving story of the RAAS in hypertension, diabetes and CV disease – moving from macrovascular to microvascular targets. Fundamental & Clinical Pharmacology. 2009; 23(6): 693-703. doi: 10.1111/j.1472-8206.2009.00780.x
- Boström K. Insights into the mechanism of vascular calcification. Am J Cardiol. 2001;88(2A): 20E-22E.
- Fraser JD, Price PA. Lung, heart, and kidney express high levels of mRNA for the vitamin K-dependent matrix Gla protein. Implications for the possible functions of matrix Gla protein and for the tissue distribution of the gamma-carboxylase. J Biol Chem. 1988; 263(23): 11033-11036.
- Wang L, Raikwar N, Deng L, et al. Altered gene expression in kidneys of mice with 2,8-dihydroxyadenine nephrolithiasis. Kidney International. 2000; 58(2): 528-536. doi: 10.1046/j.1523-1755.2000.00199.x
- Gao B, Yasui T, Itoh Y, et al. A Polymorphism of Matrix Gla Protein Gene is Associated with Kidney Stones. Journal of Urology. 2007; 177(6): 2361-2365. doi: 10.1016/j.juro.2007.01.118
- Herrmann SM, Whatling C, Brand E, et al. Polymorphisms of the Human Matrix Gla Protein (MGP) Gene, Vascular Calcification, and Myocardial Infarction. Arteriosclerosis, Thrombosis, and Vascular Biology. 2000; 20(11): 2386-2393. doi: 10.1161/01.atv.20.11.2386
- Lu X, Gao B, Liu Z, et al. A polymorphism of matrix Gla protein gene is associated with kidney stone in the Chinese Han population. Gene. 2012; 511(2): 127-130. doi: 10.1016/j.gene.2012.09.112
- Khan SR, Pearle MS, Robertson WG, et al. Kidney stones. Nature Reviews Disease Primers. 2016; 2(1). doi: 10.1038/nrdp.2016.8
- Shoag J, Tasian GE, Goldfarb DS, et al. The New Epidemiology of Nephrolithiasis. Advances in Chronic Kidney Disease. 2015; 22(4): 273-278. doi: 10.1053/j.ackd.2015.04.004
- Rendina D, De Filippo G, D’Elia L, et al. Metabolic syndrome and nephrolithiasis: a systematic review and meta- analysis of the scientific evidence. Journal of Nephrology. Published online April 3, 2014. doi: 10.1007/s40620- 014-0085-9
Supporting Agencies
Copyright (c) 2023 Anabel Abib, Elisa E. del Valle, Gustavo Laham, Francisco R. Spivacow

This work is licensed under a Creative Commons Attribution 4.0 International License.

This site is licensed under a Creative Commons Attribution 4.0 International License (CC BY 4.0).

Prof. Prakash Deedwania
University of California,
San Francisco, United States




